The next generation of Lyme + Co-Infection Diagnostics—designed to detect at every stage
The most sensitive test methodology available for direct detection of Bartonella, Babesia, and Borrelia species (spp). (BBB) pathogens.
People can be exposed to Bartonella, Babesia, and Borrelia through exposure to ticks, fleas, lice, sand flies, and potentially spiders. These vectors can carry multiple pathogens at once, making co-infections common. These stealth pathogens can cause overlapping, persistent symptoms that are often misdiagnosed. Identifying which of these infections are present helps providers tailor treatment more effectively—especially since each requires a different therapeutic approach. Without proper testing, co-infections can be missed, leading to incomplete or ineffective care.
Discover the Power of MosaicDX’s Tickborne BBB Direct Detect
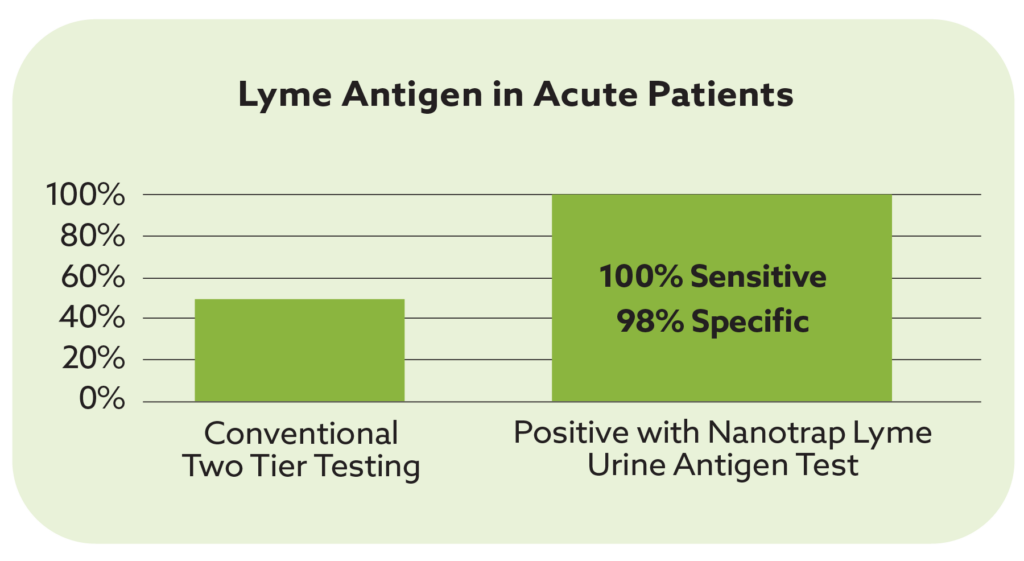
Direct testing is particularly valuable in detecting the current presence of pathogens when antibody responses might be absent or unclear. Many patients with detectable spp. DNA never develop detectable antibodies, while others may only show very low titers. The result is that many patients with current presence of pathogens are missed by both conventional PCR and antibody testing.
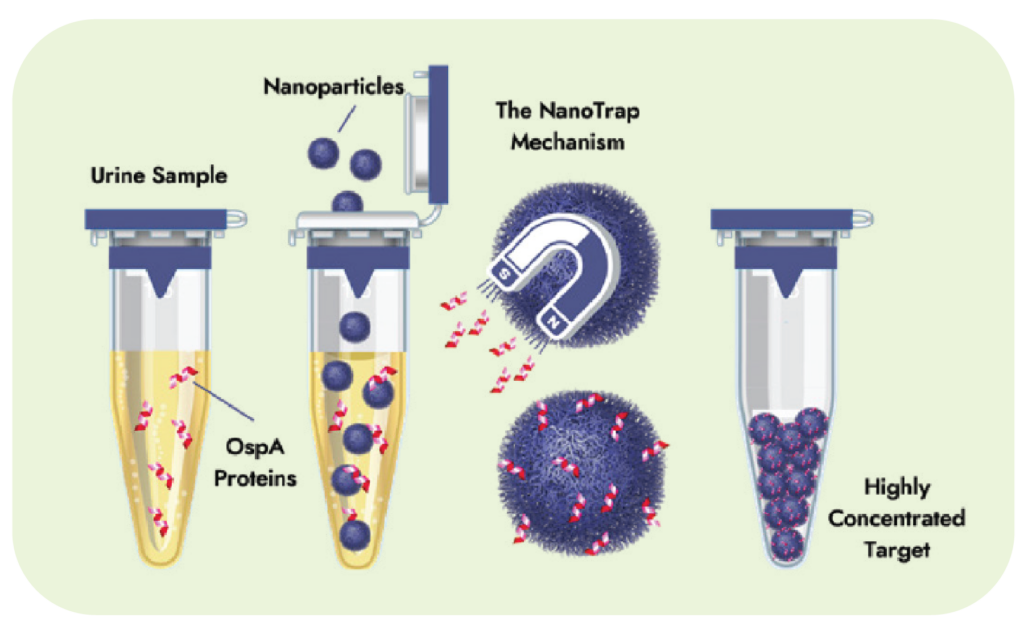
Tickborne BBB Direct Detect dPCR, confirms the presence of pathogens by directly detecting genus-level DNA for a broad range of Bartonella, Borrelia, and Babesia species (spp) in whole blood. This approach ensures that less common species are not missed.