The next generation of Lyme + Co-Infection Diagnostics—designed to detect at every stage
Our most comprehensive Lyme and co-infection combination panel includes Tickborne BBB Direct Detect dPCR with the Lyme Borrelia Nanotrap® urine antigen test for optimal coverage for the top flea and tick-borne infections at the genus level. Bartonella IgG Detect IFA adds enhances diagnostic accuracy when combined with BBB Direct Detect species genus level digital PCR Bartonella results.
Tickborne BBB Direct Detect – dPCR (1 day or 3 day) and Bartonella IgG Detect – IFA and Lyme Borrelia Direct Detect – Nanotrap
Tickborne BBB Direct Detect + Bartonella IgG Detect IFA
Tickborne BBB Direct Detect dPCR, confirms current presence of pathogens by directly detecting genus-level DNA for a broad range of Bartonella, Borrelia, and Babesia species (spp) in whole blood. This approach ensures that less common species are not missed.
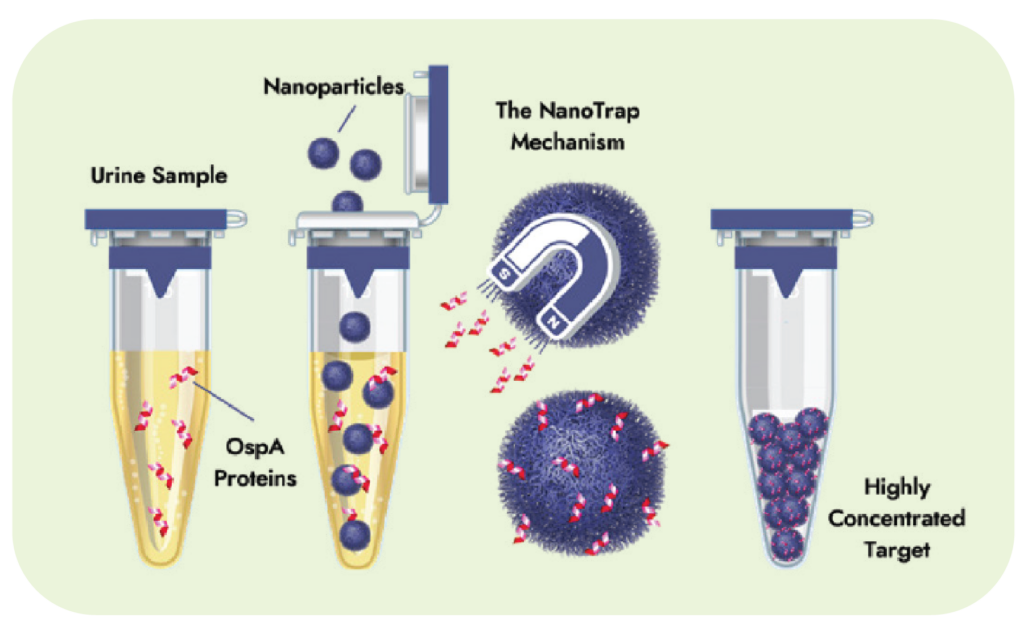
Lyme Borrelia Direct Detect – Nanotrap® assesses the current presence of Lyme Borrelia infection in urine. Concentrations of Lyme Borrelia are so low in the blood that a blood draw is unlikely to capture the pathogen in the test tube. The Nanotrap® test is targeting the OspA protein, which can be found on the surface of Borrelia species.
Bartonella IgG Detect IFA confirms antibodies against the four most common species of infection: Bartonella henselae, Bartonella koehlerae, Bartonella quintana, and Bartonella vinsonii berkhoffii.
Combining direct and indirect testing methods–specifically for Bartonella infection–enhances diagnostic accuracy, especially for patients with complex clinical presentations. MosaicDX recommends maximizing diagnostic data by testing for both antibodies (indirect) and DNA evidence of infection (direct) for Bartonella, as each test method provides critical support. Tickborne BBB Direct Detect dPCR includes tests for Borrelia, Babesia and Bartonella at a genus level for all associated pathogens and Bartonella IgG Detect uses the IFA method to detect IgG to the top four (4) Bartonella species affecting humans in North America.
We offer nine (9) individual tests and combinations of the most common Lyme and co-infections in North America, with options designed to meet every practitioner’s need.