Kurt Woeller, DO shares information about Candida albicans, the most commonly encountered human fungal pathogen that is frequently found in the digestive tract, and why host defense is dependent upon a finely tuned implementation of innate and adaptive immune responses.
SUMMARY:
Candida albicans is the most commonly encountered human fungal pathogen and is frequently found on the digestive tract. Host defense against Candida is dependent upon a finely tuned implementation of innate and adaptive immune responses, enabling the host to neutralize the invading fungus. Dr. Kurt Woeller explains how the Th1 and Th17 cells play an important role in defending the body against candida.
OVERVIEW OF THE IMMUNE SYSTEM, MUCOSAL PATHOGEN DETECTION, AND INFLAMMATION ACTIVATION
The complexity of the immune system comes from its multiple cell types and overlapping functions that coordinate in their activity to recognize, combat, and initiate acute and prolonged defenses against different pathogens. The main categories of the immune system are referred to as innate and adaptive, aka acquired. The physical barriers which include the skin and mucus membranes are often described as part of innate immunity:
- The physical barriers provide a protective defense against opportunistic and invading pathogens. If one of these barriers are breached, either from injury or inflammation, immune chemicals are produced to stop the infection. If this is not successful, then more specific innate immune cells such as monocytes are activated.
- The innate immune system includes granulocytes like basophils, neutrophils, and eosinophils, as well as other specialized cells such as monocytes and macrophages. These innate immune cells are not intrinsically affected by prior antigen exposure and typically respond similarly with each new encounter.
- The adaptive immune system, aka acquired immune system, is highly specialized to react to various pathogens. This includes the B-cell and T-cell lymphocytes. Adaptive immunity has an important immune memory capacity for pathogens of previous exposure.
Neutrophils and monocytes play an important role in innate immunity in the early stages of infection and injury. Macrophages, known as “big eaters,” are derived from circulating monocytes, and are critical for engulfing antigen which has first been recognized and processed by tissue infiltrating neutrophils. For simplicity, the focus here will be on the relationship between neutrophils and macrophages in a process of apoptotic neutrophil activation for macrophage engulfment.
When a bacteria or fungus invades a tissue such as the mucosal lining of the digestive system there is a flood of immune chemicals, i.e., cytokines and chemokines, released that signal circulating neutrophils to the site of activity. The end goal of this reaction is to destroy the invading pathogen. After the neutrophil has neutralized the pathogen, it transforms into an apoptotic neutrophil. This neutrophil form needs to be quickly cleared from the tissue before its breaks down and causes more tissue damage through release of its enzyme granules. The macrophage that is residing in the same tissue area is called into action to engulf (“eat”) the apoptotic neutrophil and then clear it through the locally draining lymph system. If this process does not occur in a timely fashion, the apoptotic neutrophil will degrade, release its toxic granules, and cause more tissue inflammation.
PRR AND PAMP
Another important aspect of innate immunity is its ability to communicate with the adaptive arm of the immune system for a systemic and more comprehensive response. This occurs, in part, through pattern recognition receptors (PRR) and pathogen associated molecular patterns (PAMP).
The immune system is linked to various tissue receptors such as those in the gastrointestinal tract containing pattern recognition receptors. Each PRR is unique to a specific pathogen associated molecular pattern. Candida contains various PAMPs unique to its cellular structure, particularly within its complex membrane layers. For example, β-glucan, part of the candida cell membrane, is a type of PAMP that our PRRs can recognize. Candida will attempt to shield the β-glucan PAMP from the host immune surveillance system. When a fungal organism such as candida contacts the epithelial surface there are various PAMPs recognizable by host cell PRRs. Uniquely, candida interacting with the epithelial cell also triggers a series of behavioral changes within the candida organism to become invasive. The PRR-PAMP interaction is crucial with regards to immune recognition and action against a pathogen.
DENDRITIC CELLS AND ANTIGEN PRESENTATION
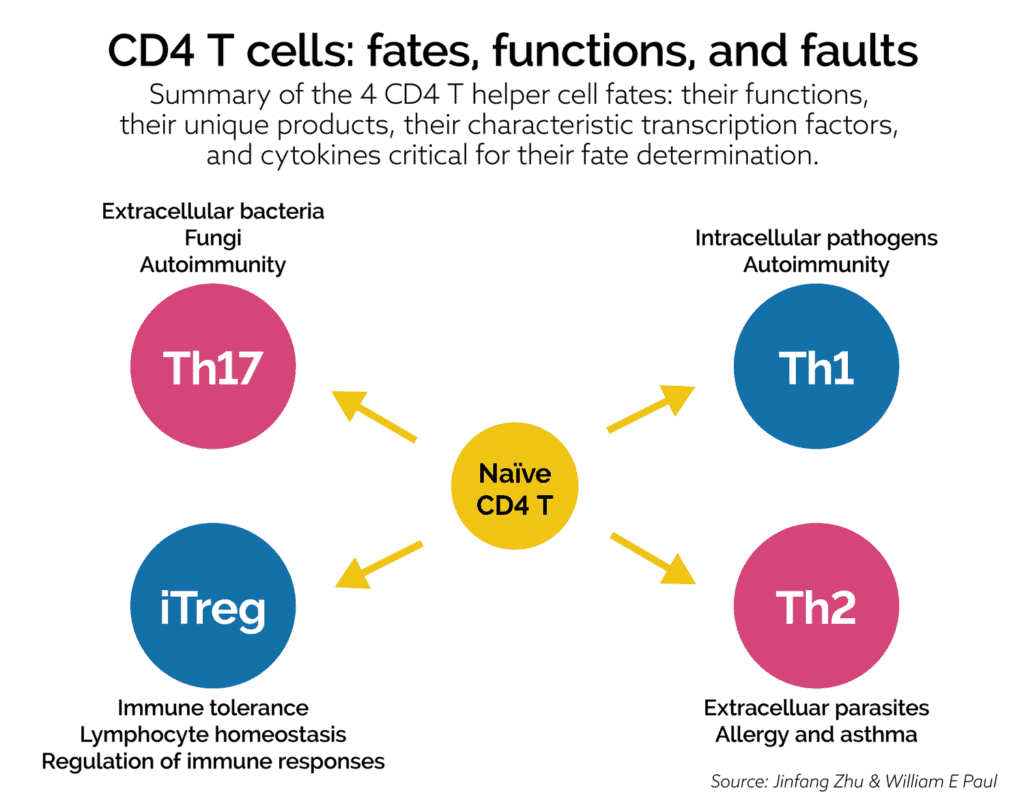
The innate immune system has additional cells called antigen presenting cells (APC). These cells relay information about a specific PAMP to the adaptive immune system. Specific APCs known as dendritic cells (DC) communicate with naïve T-cells to bring into action adaptive immune activity. This interaction between the DC and naïve T-cells induces various other T-cell types, including T-helper cells (CD4) such as Th1 and Th2, and cytotoxic T-cells (CD8). Two additional T-cell types called Th17, and T-regulatory (Treg) cells are important with regards to this discussion about candida and immune activation.
CANDIDA, AUTOIMMUNITY, AND AUTOINFLAMMATION
Autoinflammation is inflammation that occurs as a result of an autoimmune reaction. Autoimmunity is a condition that happens when the immune system attacks self-tissue. Rheumatoid arthritis and Chron’s disease are two types of autoimmune diseases where components of the immune system attack the joints and digestive system tissues, respectively, causing autoinflammation. Candida, in some cases, can be a trigger for autoimmune and subsequently autoinflammation.
T-CELL IMMUNITY PLAYS PIVOTAL ROLE FIGHTING CANDIDA
T-cells are part of the adaptive immune system. They play an important role in immune surveillance of both intracellular and extracellular pathogens, as well as immune tolerance. Here is a brief description of the actions of Th1, Th2, Th17, and Treg cells:
- Th1 cells are directed towards intracellular pathogens such as viruses. For many years imbalances in Th1 cells levels or its regulation were felt to play a major role in autoimmune reactions. This was the case until Th17 cells were discovered.
- Th2 cells are directed towards extracellular parasites and can be involved in allergy, asthma, and similar conditions.
- Th17 cells are directed towards extracellular bacteria and fungus. They are now recognized to play a significant role in autoimmunity. Th17 cells are important for mucosal reactivity against fungus, e.g., Candida albicans.
- Treg (T-regulatory) cells are regulators of overall immune responses, immune tolerance (to aid against immune attack against self-tissue), and homeostasis.
THE DANCE OF T-CELLS
The relationship between these T-cells is a complicated dance coordinated by various cytokines such as TGF-β, INF-α, and IL-6, as well as cell membrane receptors, second messenger reactions, and induction of genetic influencing transcription factors which influence protein production. As a general overview as it relates to candida and autoimmunity, the following is key information:
- Increased Th17 cells producing IL-17 against candida can have a suppressive effect on Treg.
- Decreased Treg can lead to reduced immune tolerance
- Reduced immune tolerance increases the potential for autoimmune reactions
- Reduced Treg decreases its influence on Th1 and Th2 which may also lead to increased activity of these T-cells in response to other existing pathogens.
- Non-regulated immune activity of Th1, Th2, and Th17 in various tissues can lead to increased inflammation causing tissue destruction and cellular degradation.
- Increased cellular degradation can lead to cellular structure exposure which can increase self-antigen immune recognition and drive autoimmunity.
Although there is always more to learn about the immune system and means of intervention. The next short section are some basic strategies which can be implemented for improved health, digestive function, and reduced potential for candidiasis triggering of autoimmunity.
BASIC STRATEGIES FOR REDUCING GASTROINTESTINAL CANDIDA AND POTENTIAL MUCOSAL IMMUNE ACTIVATION
The following is a short list and descriptions of things that can be helpful in improving digestive system health, microbiome diversity, and eliminating/reducing opportunistic candida:
- Microbiome diversity can be greatly enhanced through a high plant-based diet, fiber, and the use multi-strain probiotics. The improved microbiome diversity aids in pathogen reduction, mucosal barrier integrity, and reduced capacity for fungal overgrowth.
- The mucosal immune function helps to maintain optimal signaling between the mucosal immune mechanisms and innate and adaptive immune reactivity. Multi-strain probiotics, i.e., lactobacillus and bifidobacteria species, secretory IgA (SIgA) stimulators such as colostrum, Saccharomyces boulardii (which helps combat opportunistic candida and promotes SIgA production), can all be helpful.
- Elimination or reduction of opportunistic pathogens through antimicrobial support, including individual or combination botanicals.
- Elimination of gut mold colonization and mycotoxins
- Elimination or reduction of environmental chemicals known to alter immune and gut function.
- Incorporating omega-3 fatty acids (DHA & EPA), resveratrol, and curcumin to aide in inflammation control and oxidative stress.
ReferencesCandidiasis. Fungal Diseases. United States: Centers for Disease Control and Prevention. 13 November 2019.Candidiasis. cdc.gov. February 13, 2014. Archived from the original on 29 December 2014.Cottier F, Hall R. Face/Off: The Interchangeable Side of Candida albicans. 2000. Frontiers in Cellular and Infection Microbiology.Roe K. How major fungal infections can initiate severe autoimmune diseases. 2021. Microbial Pathogenesis. Science Direct.Nydiaris, H.S., et.al. Th17 Cells in Immunity to Candida albicans. 2012. Cell Host & Microbe Review.Peter J. Delves, Seamus J. Martin, Dennis R. Burton, Ivan M. Roitt. Roitt’s Essential Immunology 13th. 2017. Wiley-Blackwell Publishing.