In assessment of the Organic Acid Test (OAT), a beneficial section is the “Indicators of Detoxification,” The first marker found in this section, marker 58, gives insight into detoxification and glutathione synthesis. As this marker increases, it can guide practitioners to the possibility of toxic exposure being a piece to the client’s clinical picture. In understanding the biochemistry of this marker, further dysfunction may potentially be prevented.
Pyroglutamic Acid
Marker 58 In this section is a urinary metabolite known as pyroglutamic acid. This is a functional marker of glutathione status. Though it does not directly look at glutathione status, it measures the activity of the rate limiting enzymatic process in the production of glutathione. Thus, it is assessing the process of antioxidant production, and when upregulated informs us of increased stress on the system. Glutathione, itself, is the major antioxidant produced in abundance in the liver. It is composed of three amino acids: cysteine, glycine, and glutamate. In periods of nutritional deficiency, stress, increased reactive oxygen species (ROS), toxicity, mitochondrial dysfunction, cellular proliferation, Phase 2 detoxification, etc. glutathione stores are depleted, and the production status is upregulated. Prolonged glutathione depletion leads to mitochondrial dysfunction, that can be assessed on the OAT, and is directly supported with glutathione administration.
Glutathione Production
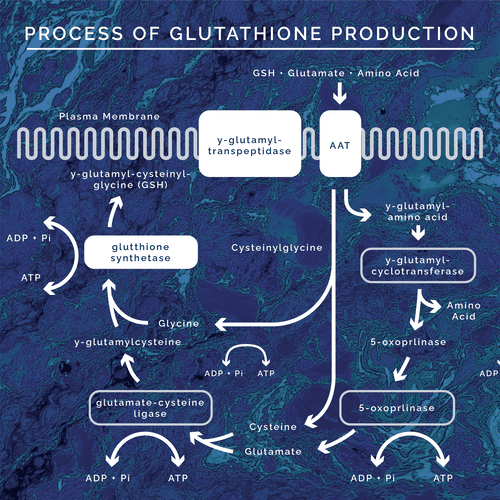
In general, the production of glutathione involves a series of enzymatic reactions that combine the three necessary amino acids for its production. On a regular basis the following process is completed to produce daily needed amounts of glutathione:
The process of glutathione production is known as the gamma-glutamyl cycle. In this cycle pyroglutamate, also known as 5-oxoproline, is degraded by the ATP dependent enzyme 5-oxoprolinase into glutamate. Glutamate is then combined with cysteine via the enzyme gamma-glutamyl cysteine synthetase. The next step is the incorporation of glycine via GSH synthetase to make glutathione itself. Glutathione is then transported out of the cytosol for use throughout the body. For the cycle to continue, reduced glutathione is transported back into the cytosol. In order to be transported back into the cell, it must be enzymatically cleaved by gamma glutamyl transpeptidase (GGT- another significant blood biomarker for glutathione status) to break it down into a cysteine-glycine complex and free glutamate. The cysteine-glycine complex is then cleaved further into the respective amino acids then transported into the cell. The glutamate is then transported into the cell as gamma-glu-aa and is transformed into 5-oxoproline via gamma glutamyl cyclotransferase. From here it can then be cleaved into glutamate via 5-oxoprolinase, continuing the cycle. This process is always happening at some extent regularly in the human body.
Negative Feedback Loop
During this process, as glutathione is produced, there is a negative feedback loop. When in abundant supply, glutathione inhibits the gamma-glutamyl cysteine synthetase enzyme. This blocks the combining of cysteine and glutamate before being joined with glycine to form more glutathione. This is the body’s way of regulating the amount of glutathione in the system. In times of oxidative stress, toxic exposure, inflammation, etc the glutathione production cycle is upregulated due to an increased need for glutathione to protect the body. When glutathione is deficient, the negative feedback loop is dysregulated allowing for more cysteine to be joined in the production of more glutathione. Since the regulation is no longer happening the entire cycle of production is upregulated. Thus, there is also an upregulation of 5-oxoproline being converted into glutamate. Because the enzyme, 5-oxoprolinase, is ATP dependent and the rate limiting enzyme of the whole cycle there becomes a buildup in pyroglutamic acid. This is because the need for glutathione production, in times of stress and toxicity, outweighs the rate in which 5-oxoprolinase can transform 5-oxoproline into glutamate. So, 5-oxoproline builds up leading to elevated urinary values of pyroglutamic acid. Also, due to the ATP dependent nature of the enzyme, energy depletion is seen and stimulates the expression of energy depletion symptoms.
When evaluating marker 58 on the OAT, elevations give insight to how upregulated the glutathione cycle is at the moment of testing. In instances of true elevations, out of the reference range, there is clear glutathione deficiency. It can be said that the person’s body is struggling to make adequate glutathione. In situations like this all aspects of the client’s health presentation and medications/supplements should be evaluated. Certain pharmaceuticals, like large amounts or regular small doses of acetaminophen, which is known for its hepatotoxicity, stimulate the need for more glutathione. This is especially significant when people are taking multiple pharmaceuticals that may be liver toxic and have a cumulative effect. Other things that deplete glutathione include alcohol, recreational drug use, a fructose rich diet, the natural aging process, prolonged fasting (24 hours or longer), and anything that causes increases in oxidative stress. If this has been ruled out as the cause of elevations in pyroglutamic acid, then toxic exposures can be assessed. As stated, glutathione production is upregulated during a toxic exposure. Since the body upregulates glutathione for various reasons, it’s hard to pinpoint which toxin test to perform. It could be heavy metals, non-metal toxins, mold toxins, or a combination. Consider running Mosaic Diagnostics offered profiles: MycoTOX, TOXDetect Profile®, Heavy Metal Testing.
Assessing for depleting causes is helpful in the healing process as it can open up people to environmental testing and detoxification. Pyroglutamic acid is a useful marker and upregulation can give insight to glutathione needs.
References1. CG. Alfafara, A., CG. Alfafara, K., Anderson, M., A. Anschau, L., M. Ask, V., A. Ayer, C., . . . X. Zhang, H. (1992, January 01). Microbial production of glutathione. Retrieved December 11, 2020, from HERE2. Francini F;Castro MC;Schinella G;García ME;Maiztegui B;Raschia MA;Gagliardino JJ;Massa ML;. (n.d.). Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Retrieved December 11, 2020, from HERE3. M;, E. (n.d.). Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): A tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Retrieved December 11, 2020, from HERE4. M;, E. (n.d.). Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): A tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Retrieved December 11, 2020, from HERE5. Martensson, J., & Meister, A. (1989). Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proceedings of the National Academy of Sciences, 86(2), 471-475. doi:10.1073/pnas.86.2.4716. Pizzorno, J. (2014, February). Glutathione! Retrieved December 11, 2020, from HERE7. Richman, P., & Meister, A. (1975, February 25). Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. Retrieved December 11, 2020, from HERE8. An Under Recognised Cause of Metabolic Acidosis. (2018, September 13). Retrieved December 11, 2020, from HEREVogt, B., & Richie, J. (2002, November 05). Fasting-induced depletion of glutathione in the aging mouse. Retrieved December 11, 2020, from HERE




