A previous blog covered 8 of the common binders for mycotoxins. This blog expands upon that and covers several additional binders that research has shown can be effective on mycotoxins.
In the world of mold and mycotoxins there is an ever-evolving conversation about which binders are the best. On the one hand this conversation can be challenging since there is a vast amount of information thrown at us and it can be difficult to sift through. On the other hand, because there is so much information, we have boundless resources to support our clients that are plagued by mycotoxin exposure. In a previous post, we laid out some common binding agents and their affinity to various mycotoxins. This time the goal is to explore some other binding agents available and the research surrounding them.
When it comes to mycotoxin binding research, we must keep in mind the toxic nature of these toxins. Their toxicity makes the research limited to animals, animal feed, and in vitro studies. Due to the unethical nature of purposefully exposing humans to mycotoxins, there is limited research on these binders in humans. This is acceptable as the binding capacity can still be evaluated efficiently. In your journey to understanding how our bodies process mycotoxins it is crucial to understand their metabolism. Checkout theses previous posts that describe the metabolism of the mycotoxins analyzed by Mosaic Diagnostics: Mycotoxin Properties and Metabolism, Part 1 and Part 2.

OKRA AND OTHER VEGETABLES
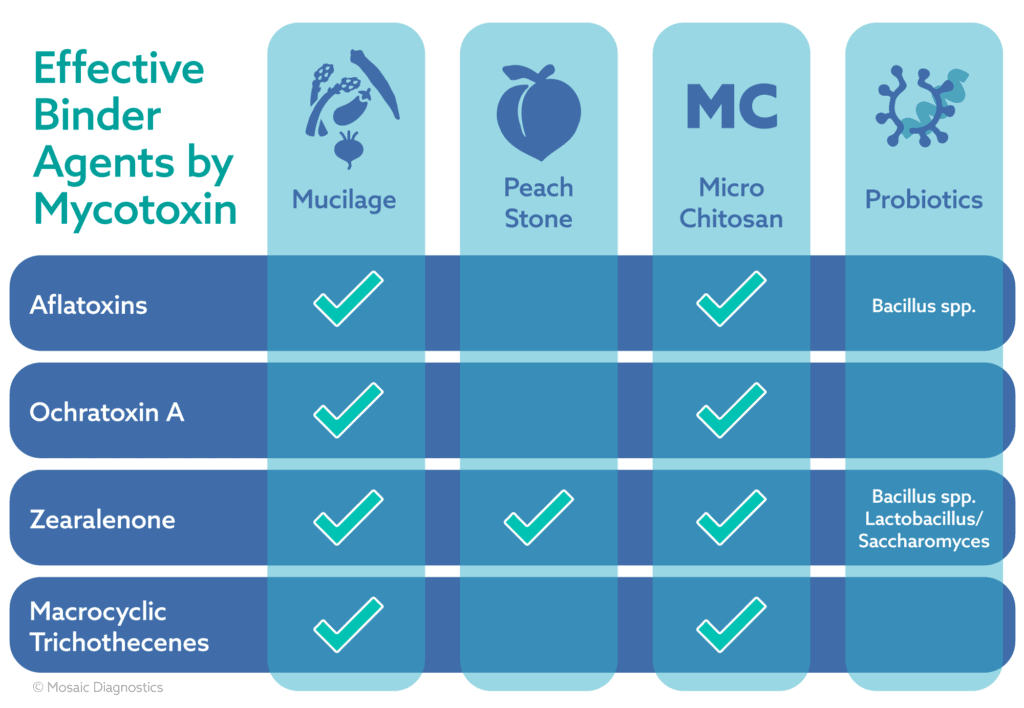
The first binding agent is a well-known property of certain vegetables. The mucilaginous nature of foods like okra are what give them their binding power. A mucilage is a sticky, slimy, vicious type of glue that is polysaccharide rich. It is often activated when wet. Think of the gel that is produced when you soak flaxseeds in water5. This gelatinous, gluey nature makes okra and other vegetables able to bind bile acids in the gastrointestinal tract that are carrying toxins. Okra, when studied, had the highest binding capacity to bile and was followed by beets, asparagus, and eggplant. Turnips, green beans, carrots, and cauliflower had less binding capacity than the others, but they are equal to each other. The binding capacity was measured in relation to the capacity of cholestyramine.
Okra was shown to bind at 15 percent the capacity of cholestyramine12. Though this may seem small, this type of binding capacity is from food. This is huge! With this information we have more science to back up the benefits of eating certain plant foods as regular staples in your diet. Supplements can be used as well. Since there is a lower binding capacity and most of these foods are fibrous this could be a good option for our constipated clients when it comes to mycotoxin binding.

PEACH STONE
This next adsorbent option is one I had not initially thought about. It’s peach stone. The pit of a peach has a composition of biological polymers like cellulose, lignin, and hemicellulose. These compounds increase the intensity of the hydroxyl and phenol groups which give it the adsorbing quality6. Additionally, the substantial structure of the peach stone, with all its openings and canals, also makes for a larger surface area for binding sites. By studying both modified and unmodified peach stone (MPS/PS) we know that this binder has the capacity to bind aflatoxin B1, ochratoxin A (OTA), deoxynivalenol (DON), zearalenone (ZON), diacetoxyscirpenol (DAS) and T-2 toxin. Both MPS and PS, close to physiologic pH of 7.0, had specific affinities to each toxin6. PS had a higher binding capacity for aflatoxin B1 than MPS, while both had equal binding capacity to ochratoxin and T-2 toxin. MPS had higher affinity for DON, ZON, and DAS as compared to PS6. Overall, the MPS higher the most consistent binding affinity and would be a good overall binder for multiple toxin elevations.

MICRO CHITOSAN
Micro chitosan is another commonly used product. It is a polysaccharide from the outer shell of shellfish like crab and shrimp. It is traditionally used in drug and medicine manufacturing13. As of late, it has been studied as a binding agent in poultry and in corn and wheat crops to reduce mycotoxins. One study showed that chitosan binds Fusarium toxins, fumonisins and DON, in corn and wheat products14. Another revealed in a poultry gastrointestinal model, that chitosan has a wide binding capacity. In this study chitosan bound to, in increasing order, AFB1, FB1,FB2, OTA, AFG2, AFG1, ZEA and AFB2 2,4. Another study also linked chitosan as a good adsorbent to trichothecenes11. Not many binders have been linked to trichothecenes, so this is a benefit to clients that are unfortunately exposed to these. As a side note goji berries have been linked with charcoal as a a winning pair in binding and decreasing toxic effects of T2 toxin10. With micro chitosan take caution and avoid use in anyone who has a shellfish allergy.
PROBIOTICS
In the first installment of this topic, probiotics were highlighted for their ability to bind certain mycotoxins and to discourage mold organism’s growth. Discussed were probiotics such as Saccharomyces and Lactobacillus species and their ability to bind toxins like citrinin and zearalenone. Sweeping the functional medicine community is the use of spore or soil-based probiotics. Their efficacy in diversifying the gut microbiome is well documented. This type of probiotic has also been studied as a mycotoxin binder. B. subitillus has been documented to degrade aflatoxin B1 by 76%, zearalenone by 84%, and deoxynivalenol by 78%. Along with other bacillus species (B. lincheniformis, B. megaterium, B. cereus, and B. thuringiensis) these spore base probiotics are efficacious in binding mycotoxins with the addition of manganese9. The Mn2+ is proposed to work with the B. subitillus organism to degrade mycotoxins through manganese peroxidase. Another species, B. coagulans, has been shown to inhibit the production of DON from Fusarium mold1. Other bacillus species have been shown to bind and inactivate other mycotoxins like T-2 and patulin 8. T-2 is also bound by Lactobacillus and Saccharomyces species7. This information shows us that a variety of probiotics are useful in the supportive care of those with mycotoxin exposure.
These insights are beneficial for those that may not respond well to other binders. Highlighted are options that are easily accessed. Some of these can be incorporated into a healthy diet. This will not only add an adsorption capacity but also nutritional value. With more options of effective binder agents practitioners have more tools in their toolboxes and client have more support where they need it.
References
- A;, C. K. K. (n.d.). Antifungal activity of bacillus coagulans against Fusarium SP. Acta microbiologica Polonica. https://pubmed.ncbi.nlm.nih.gov/12588102/
- Abbasi Pirouz, A., Selamat, J., Zafar Iqbal, S., & Iskandar Putra Samsudin, N. (2020, February 12). Efficient and simultaneous chitosan-mediated removal of 11 mycotoxins from Palm Kernel Cake. MDPI. https://www.mdpi.com/2072-6651/12/2/115
- Adhikari, M., Negi, B., Kaushik, N., Adhikari, A., Al-Khedhairy, A. A., Kaushik, N. K., & Choi, E. H. (2017, May 16). T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5464924/
- Delgado-Cedeño, A., Hernández-Martínez, S. P., Ramos-Zayas, Y., Marroquín-Cardona, A. G., Méndez-Zamora, G., Franco-Molina, M. A., & Kawas, J. R. (2022, November 10). Insoluble chitosan complex as a potential adsorbent for aflatoxin B1 in Poultry Feed. Frontiers. https://www.frontiersin.org/articles/10.3389/fmats.2022.1044495/full
- A grammatical dictionary of botanical latin. MO Garden main page. (n.d.). http://www.mobot.org/mobot/latindict/keyDetail.aspx?keyWord=mucilaginous
- In vitro evaluation of the efficacy of Peach Stones as … – doiserbia. (n.d.). http://www.doiserbia.nb.rs/img/doi/0352-4906/2013/0352-49061324287L.pdf
- Janik, E., Niemcewicz, M., Podogrocki, M., Ceremuga, M., Stela, M., & Bijak, M. (2021, November 14). T-2 toxin-the most toxic trichothecene mycotoxin: Metabolism, toxicity, and decontamination strategies. MDPI. https://www.mdpi.com/1420-3049/26/22/6868
- Piotrowska, M. (2021, November 19). Microbiological decontamination of mycotoxins: Opportunities and limitations. MDPI. https://www.mdpi.com/2072-6651/13/11/819
- Qin, X., Su, X., Tu, T., Zhang, J., Wang, X., Wang, Y., Wang, Y., Bai, Y., Yao, B., Luo, H., & Huang, H. (2021). Enzymatic degradation of multiple major mycotoxins by dye-decolorizing peroxidase from bacillus subtilis. Toxins, 13(6), 429. https://doi.org/10.3390/toxins13060429
- Scientific Research Publishing. (2014, December 1). Evaluation of gojiextract and charcoal as antioxidant on T-2 toxin administration onliver male mice. Food and Nutrition Sciences. https://www.scirp.org/html/3-2701332_52009.html
- Solís-Cruz, B., Hernández-Patlán, D., Beyssac, E., Latorre, J. D., Hernandez-Velasco, X., Merino-Guzman, R., Tellez, G., & López-Arellano, R. (2017, October 19). Evaluation of chitosan and cellulosic polymers as binding adsorbent materials to prevent aflatoxin B1, Fumonisin B1, ochratoxin, Trichothecene, deoxynivalenol, and Zearalenone Mycotoxicoses through an in vitro gastrointestinal model for poultry. Polymers. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6418884/
- Trowell, H. C., Suckling, K. E., Story, J. A., Rossi, S. S., Kritchevsky, D., Kahlon, T. S., Hofmann, A. F., Hecht, S. S., Gautam, M., Eastwood, M. A., Diwanay, S., Daggy, B. P., Carey, M. C., Balmer, J., Ames, B. N., & Anderson, J. W. (2006, October 6). In vitro binding of bile acids by okra, beets, asparagus, eggplant, turnips, green beans, carrots, and cauliflower. Food Chemistry.
- WebMD. (n.d.). Chitosan: Overview, uses, side effects, precautions, interactions, dosing and reviews. WebMD. https://www.webmd.com/vitamins/ai/ingredientmono-625/chitosan
- Zachetti VGL;Cendoya E;Nichea MJ;Chulze SN;Ramirez ML; (n.d.). Preliminary study on the use of Chitosan as an eco-friendly alternative to control fusarium growth and mycotoxin production on maize and wheat. Pathogens (Basel, Switzerland). https://pubmed.ncbi.nlm.nih.gov/30841490/