Binding agents are an integral part of mycotoxin illness detoxification. Trying to decide which binder is correct for which mycotoxin can be a difficult process. This blog provides a collection of research connecting mycotoxins to a good binder choice.
After a positive Organic Acids Test and MycoTOX Profile, the presence of mold and mycotoxins are usually significant answers for many symptomatic clients. When working with mycotoxicosis, choosing the correct binder can present a challenge. Oftentimes I get the question: which binder is correct for which toxin? Since the research for the binding capacity of each binder isn’t as heavily researched as other agents, it can be daunting to sift through the information. Below is a collection of research connecting mycotoxins to a good binder choice.
In our bodies, toxins are detoxed and excreted through a few pathways. Routes of elimination include urine, stool, bile and through our skin. Other routes include tears and saliva but are negligible in the realm of detoxification. Another route is breast milk. Since breast milk is a route of excretion this means toxins can be transferred to another life this way. This fact makes binders even that much more crucial in childbearing-age women.
When it comes to binders, bile and stool are the target routes of elimination. Fat-soluble substances such as dietary lipids, certain vitamins and fat-soluble toxins like mycotoxins get packaged into bile for absorption and detoxification. During bile’s life cycle it gets excreted into the GI tract and is what gives stool its brown color. In the colon most of the bile is reabsorbed so the liver and gallbladder do not have to work as hard to make more bile. It is recycled and reused. Dysfunction of this phenomenon, bile acid malabsorption, chronic diarrhea is the main symptom. Since bile is reabsorbed, in the ileum and jejunum, if toxins are packaged in the bile then the toxins can be reabsorbed as well. They would then re-enter circulation via the hepatic portal system. This is where binders come in handy. Binders will adhere to the bile that packages the toxins and then it cannot be reabsorbed. Due to the nature of this adherence, it cannot be trusted that the bond is irreversible. This bond is more like static cling, as described by Dr. Neil Nathan. The lack of a tight bond allows for the bile to be released if not excreted regularly. Meaning, irregular bowel movements from constipation, lack of fiber, or motility issues could cause resorption of toxins even with binder usage. Binders by nature are constipating and this should be mitigated and assessed regularly during binder usage. Properly moving bowels through diet and supplements should be achieved prior to adding in any binding agent.
Cholestyramine / Welchol
In the world of prescriptions, cholestyramine and colesevelam hydrochloride, more commonly known as Welchol, are often binders of choice. They are known for their intended use and design, which is their lipid lower activity and use in glycemic control in patients with type 2 diabetes mellitus. They work by directly binding bile in the GI tract. This causes a reduced bile resorption and an increased conversion of cholesterol to bile, via 7a-hydroxylation, thus lowering cholesterol levels. In this process toxin laden bile is bound and thus excreted via stool.
These two binders are often used in mycotoxin detoxification protocols. As seen above, this is for good reason. The mycotoxin that responds best to these prescriptions is ochratoxin a (OTA), according to the research. This is a notorious mycotoxin. OTA is produced by many species of Apergillus and Penicillium molds. These are two of the most ubiquitous molds in the environment. This fact makes OTA the most common mycotoxin. It is so common that even regular ingestion of commonly moldy foods will most likely expose you to small, negligible amounts of OTA – we outline this in our article: Mycotoxins in Food. In cases of water damage building exposure these drugs are valuable assets to binding this mycotoxin.
Charcoal
A few years ago, activated charcoal was the new buzz trend. It was popping up in drinks, snacks, even ice cream. Due to this most people are familiar with this binder. It is commonly used for firming loose stools, binding toxin from food poisoning, and now for mycotoxin binding. According to the research most binders will bind to just about anything, including nutrients necessary for life. They are non-discriminating. This fact made the charcoal trend rather troubling for those engaging in high intake of this substance with no regard to its potential danger if not taken responsibly. But, due to this, activated charcoal is an effective toxin binder to just about any toxin that is excreted in the gut. It works similarly to cholestyramine by adsorbing to toxins packaged in bile.
Research shows that in food stuff and in the body activated charcoal is beneficial in mycotoxin binding. OTA is bound effectively by charcoal products. This a good alternative for non-prescribing practitioners. Charcoal has also shown efficacy in adsorbing to macrocyclic trichothecenes. Two of the most common are verrucarin a and roridin e, and these are assessed on the MycoTOX Profile. Other subvarieties of verrucarin, including ‘J’ have shown binding efficacy with charcoal administration. Also, T-2 toxins from fusarium are bound by charcoal.
Clay
Another common binding agent is bentonite clay and zeolite clay. These two clays have been touted as working wonders in the cosmetic arena by pulling toxins from the skin. These clays have been shown to bind greatly to toxins in animal feed, reducing the toxic load before consumption. Another clay is montmorillonite clay, also known as Novasil. This clay has ample research as a mycotoxin adsorbent in animal feed, a highly mycotoxin contaminated source. Great news, these clays do the same adsorbing action in the GI tract. Both agents show great affinity for binding aflatoxins best. These clays do not have much other research connecting them as strong adsorbents to other toxins as they do with aflatoxins. Although they can be useful in clients with these toxins and zearalenone, OTA, and gliotoxin as there is some adsorbing activity with these toxins.
Glucomannan
This water-soluble polysaccharide is a well-touted weight loss solution. It comes from the elephant yam, konjac. It is a hemicellulose fiber with beta-D-glucose and beta-D-mannose with acetyl groups with beta 1–4 linkages. Due to the lack of enzymes in human saliva to break these linkages, glucomannan goes through the GI tract unchanged. This allows for it to bind without absorption. Due to its content, this fiber can adsorb up to 50 times its weight. Glucomannan has shown efficacy in binding various mycotoxins. Aflatoxin and OTA are major toxins affected by this binder. Others include zearalenone and Toxin T-2. not much efficacy was seen in binding DON-1 (Deoxynivalenol). Since glucomannan is a fiber this may be an option to consider in more constipated clients with ample water intake.
Fiber
Speaking of fiber, in general fibrous supplements and foods can act as simple overall binders. Fiber from oats, wheat bran, alfalfa, lignans in flax and chia, guar gum, etc have been used as early interventions in lowering cholesterol. It has the same effect that cholestyramine has on cholesterol. It is due to the bile sequestering activity of these fibers that work to lower cholesterol. In turn this will also lower toxic load. Even though fiber doesn’t have much direct research in the binding of specific mycotoxins, it is always a good dietary change to implement. Barley and oats showed highest absorptive capacity amongst other fibers when tested. Another great fiber binder to consider is modified citrus pectin (MCP). This binder has shown great efficacy in binding heavy metals such as lead. Though this isn’t a mycotoxin, this shows us that MCP has a great potential utility in any detox protocol.
Chlorella
This next binding agent is a common plant-based agent. Chlorella is a type of freshwater algae that is packaged into a tablet, liquid extracts, and powders. It is often touted as a superfood due to its highly nutritious profile. It is high in protein, vitamins A, C, and E and is a great source of fiber. Because of its nutrition, chlorella is known for its wound healing, anti-cancer, anti-aging, and immune-boosting potential. In breastfeeding mother’s chlorella intake increased circulating immunoglobulins in breast milk. This plant is great as a heavy metal binder and as a binder of aflatoxins. It has even been shown to inhibit aflatoxin B1 induced liver cancer.
Due to the safety profile of chlorella, it is a great binder for all populations. It is difficult to detox a constipated child or a woman expecting a child and is planning to breastfeed. Since so many other binders bind not only toxins, but also nutrients it can be difficult to support detox. Adding in small doses of chlorella is a safe and effective way to add in supportive detox without stimulating too much toxin release to the unborn fetus or breastfeeding child.
Humic Acid
Humic acid and its related counterpart, fulvic acid, are the final products of decomposition of organic matter. They are formed through humification of plant and animal matter via biologic processes of microorganisms. This byproduct acts as an adsorbent in soil to bind to toxic substances. Agriculturalists use these substances as soil additives to boost the growth and health of their crop due to its concentrated amount of nutrients. Due to its rapid lifecycle humic acid doesn’t compete for nutrients with the plant or any other organism that uses it. This makes this biotoxin binder simpler to utilize when taking a variety of nutritional supplements.
Not only is humic acid a great biotoxin binder, but it also has shown efficacy as an anti-inflammatory. It also has shown promise in stimulating apoptosis in promyelocytic leukemia cells. This substance, along with fulvic acid, is a wonderful well-rounded addition to any detoxification protocol.
Probiotics
This section of biotoxin binders may come as a surprise. Probiotics are best known for their activity in repopulating the GI microbiome after antibiotic use, killing of pathogenic organisms like C. difficile, and as a support in a whole host of chronic diseases. What many do not realize is that these organisms can also directly bind to mycotoxins. Strain of lactobacillus work to directly bind aflatoxins especially the B1 variety. The specific strains are L. pentosus and L. beveris. Another promising strain is L. plantarum C88. This strain works not only to bind to aflatoxins, but it also works by upregulating the antioxidant activity of glutathione s-transferase. It also shows great binding capacity to the common mycotoxin, sterigmatocystin.
Strains of saccharomyces also work well to bind mycotoxins. S. cerevisiae has been shown to bind tightly to aflatoxins. It has also shown great efficacy to bind to OTA and zearalenone. Saccharomyces boulardii, clinically, has shown great efficacy against gliotoxin. It has also shown efficacy in reducing Aspergillus and Fusarium molds in the GI tract. This will indirectly reduce mycotoxins, as it is reducing the producers of mycotoxins including zearalenone, enniatin b, OTA, gliotoxin, and aflatoxins. Mannan oligosaccharides (MOS) are prebiotics derived from the outer cell wall of S. cerevisiae. This prebiotic has been shown to bind to citrinin, which a wide variety of molds produce. Using a variety of these strains will round out not only your gut treatment but also the detoxification process.
All in all, binding agents are an integral part of mycotoxin illness detoxification. Whether someone has 1 or 10 mycotoxins populate on the MycoTOX Profile, having the correct binders can be a challenge. Hopefully, this can be used as a resource to guide you in planning toxin binder regimens to best help your clients. Using a combination of binding agents will allow for well-rounded binding capacity in any mycotoxin toxicity case. View a wide variety of binding agents at New Beginnings Nutritionals to support you in choosing the best biotoxin binders.
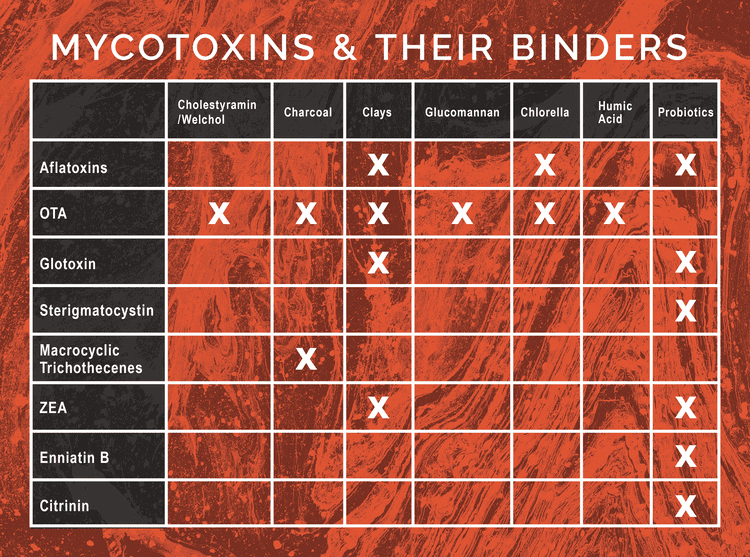
This is a table matching mycotoxin with binders that have research to their binding affinity
References
1. Agawane, S. B., and P. Lonkar. “Effect of Probiotic Containing Saccharomyces Boulardii on Agawane, S. B., and P. Lonkar. “Effect of Probiotic Containing Saccharomyces Boulardii on Experimental Ochratoxicosis in Broilers: Hematobiochemical Studies.: Semantic Scholar.” Undefined, 1 Jan. 1970, SOURCE.
2. Ardeshir Mohaghegh,Mohammad Chamani,Mahmoud Shivazad,Ali Asghar Sadeghi &Nazar Afzali. “Effect of Esterified Glucomannan on Broilers Exposed to Natural Mycotoxin-Contaminated Diets.” Taylor & Francis, SOURCE.
3. Armando, M.R., et al. “Adsorption of Ochratoxin A and Zearalenone by Potential Probiotic Saccharomyces Cerevisiae Strains and Its Relation with Cell Wall Thickness.” Journal of Applied Microbiology, vol. 113, no. 2, 2012, pp. 256–264., doi:10.1111/j.1365-2672.2012.05331.x.
4. “Chlorophyll and Chlorophyllin.” Linus Pauling Institute, 1 Jan. 2021, SOURCE.
5. D. Lloyd-Jones, R. Adams, et al. “Impact of Daily Chlorella Consumption on Serum Lipid and Carotenoid Profiles in Mildly Hypercholesterolemic Adults: a Double-Blinded, Randomized, Placebo-Controlled Study.” Nutrition Journal, BioMed Central, 1 Jan. 1970, SOURCE.
6. De Mil, Thomas, et al. “Characterization of 27 Mycotoxin Binders and the Relation with in Vitro Zearalenone Adsorption at a Single Concentration.” Toxins, MDPI, 5 Jan. 2015, SOURCE.
7. Devreese M;Girgis GN;Tran ST;De Baere S;De Backer P;Croubels S;Smith TK; “The Effects of Feed-Borne Fusarium Mycotoxins and Glucomannan in Turkey Poults Based on Specific and Non-Specific Parameters.” Food and Chemical Toxicology : an International Journal Published for the British Industrial Biological Research Association, U.S. National Library of Medicine, SOURCE.
8. El Khoury, Rhoda, et al. “OTA Prevention and Detoxification by Actinobacterial Strains and Activated Carbon Fibers: Preliminary Results.” MDPI, Multidisciplinary Digital Publishing Institute, 24 Mar. 2018, SOURCE.
9. Garcia Diaz, Tatiana, et al. “Use of Live Yeast and Mannan-Oligosaccharides in Grain-Based Diets for Cattle: Ruminal Parameters, Nutrient Digestibility, and Inflammatory Response.” PloS One, Public Library of Science, 14 Nov. 2018, SOURCE.
10. Guo M;Hou Q;Waterhouse GIN;Hou J;Ai S;Li X; “A Simple Aptamer-Based Fluorescent Aflatoxin B1 Sensor Using Humic Acid as Quencher.” Talanta, U.S. National Library of Medicine, SOURCE.
11. Hamidi, Adel, et al. “The Aflatoxin B1 Isolating Potential of Two Lactic Acid Bacteria.” Asian Pacific Journal of Tropical Biomedicine, vol. 3, no. 9, 2013, pp. 732–736., doi:10.1016/s2221-1691(13)60147-1.
12. Hope, Janette. “A Review of the Mechanism of Injury and Treatment Approaches for Illness Resulting from Exposure to Water-Damaged Buildings, Mold, and Mycotoxins.” The Scientific World Journal, Hindawi, 18 Apr. 2013, SOURCE.
13. J;, Santos RR;Vermeulen S;Haritova A;Fink-Gremmels. “Isotherm Modeling of Organic Activated Bentonite and Humic Acid Polymer Used as Mycotoxin Adsorbents.” Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, U.S. National Library of Medicine, SOURCE.
14. Jay Y. Jacela, DVM; Joel M. DeRouchey, PhD; Mike D. Tokach, PhD; Robert D. Goodband, PhD; Jim L. Nelssen, PhD; David G. Renter, DVM, PhD; Steve S. Dritz, DVM, PhD. Fact Sheet: Mold Inhibitors, Mycotoxin Binders, and Antioxidants, SOURCE.
15. Jubert, Carole, et al. “Effects of Chlorophyll and Chlorophyllin on Low-Dose Aflatoxin B(1) Pharmacokinetics in Human Volunteers.” Cancer Prevention Research (Philadelphia, Pa.), U.S. National Library of Medicine, Dec. 2009, SOURCE.
16. Kerkadi A;Barriault C;Tuchweber B;Frohlich AA;Marquardt RR;Bouchard G;Yousef IM; “Dietary Cholestyramine Reduces Ochratoxin A-Induced Nephrotoxicity in the Rat by Decreasing Plasma Levels and Enhancing Fecal Excretion of the Toxin.” Journal of Toxicology and Environmental Health. Part A, U.S. National Library of Medicine, SOURCE.
17. Kraljević Pavelić, Sandra, et al. “Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in Vivo.” Frontiers in Pharmacology, Frontiers Media S.A., 27 Nov. 2018, SOURCE.
18. Kumar, C. B. ; Reddy, B. S. V. ; Gloridoss, R. G. ; Prabhu, T. M. ; Suresh, B. N. “ Effect of MOS Based Toxin Binder on Low Level Citrinin Toxicity in Commercial Broilers.” Mysore Journal of Agricultural Sciences, vol. 48, no. 1, 2014, pp. 75–82.
19. L,Haus M;Žatko D;Vašková J;Vaško. “The Effect of Humic Acid in Chronic Deoxynivalenol Intoxication.” Environmental Science and Pollution Research International, U.S. National Library of Medicine, SOURCE.
20. Lauterburg BH, Dickson ER, Pineda AA, Carlson GL, Taswell HF. “Removal of Bile Acids and Bilirubin by Plasmaperfusion of U.S.P. Charcoal-Coated Glass Beads.” Europe PMC, 30 Sept. 1979, SOURCE.
21. Li, Yan, et al. “Research Progress on the Raw and Modified Montmorillonites as Adsorbents for Mycotoxins: A Review.” Applied Clay Science, Elsevier, 30 July 2018, SOURCE.
22. Naumann, Susanne, et al. “In Vitro Interactions of Dietary Fibre Enriched Food Ingredients with Primary and Secondary Bile Acids.” Nutrients, vol. 11, no. 6, 2019, p. 1424., doi:10.3390/nu11061424.
23. Riaz, Sana. “Cholestyramine Resin.” StatPearls [Internet]., U.S. National Library of Medicine, 25 May 2020, SOURCE.
24. Rotter, R G, et al. “Influence of Dietary Charcoal on Ochratoxin A Toxicity in Leghorn Chicks.” Canadian Journal of Veterinary Research = Revue Canadienne De Recherche Veterinaire, U.S. National Library of Medicine, Oct. 1989, SOURCE.
25. Vahouny, George V., et al. “Dietary Fibers: V. Binding of Bile Salts, Phospholipids and Cholesterol from Mixed Micelles by Bile Acid Sequestrants and Dietary Fibers.” Lipids, vol. 15, no. 12, 1980, pp. 1012–1018., doi:10.1007/bf02534316.
26. “Verrucarin A (T3D3720).” T3DB, SOURCE.
27. S,Baker; W,Shaw. “Case Study: Rapid Complete Recovery From An Autism Spectrum Disorder After Treatment of Aspergillus With The Antifungal Drugs Itraconazole And Sporanox.” Integrative Medicine (Encinitas, Calif.), U.S. National Library of Medicine, SOURCE.
28. Wang JS;Luo H;Billam M;Wang Z;Guan H;Tang L;Goldston T;Afriyie-Gyawu E;Lovett C;Griswold J;Brattin B;Taylor RJ;Huebner HJ;Phillips TD; “Short-Term Safety Evaluation of Processed Calcium Montmorillonite Clay (NovaSil) in Humans.” Food Additives and Contaminants, U.S. National Library of Medicine, SOURCE.
29. Yang, Hsin-Ling, et al. “Humic Acid Induces Apoptosis in Human Premyelocytic Leukemia HL-60 Cells.” Life Sciences, Pergamon, 25 June 2004, SOURCE.
30. Zhao ZY;Liang L;Fan X;Yu Z;Hotchkiss AT;Wilk BJ;Eliaz I; “The Role of Modified Citrus Pectin as an Effective Chelator of Lead in Children Hospitalized with Toxic Lead Levels.” Alternative Therapies in Health and Medicine, U.S. National Library of Medicine, SOURCE.