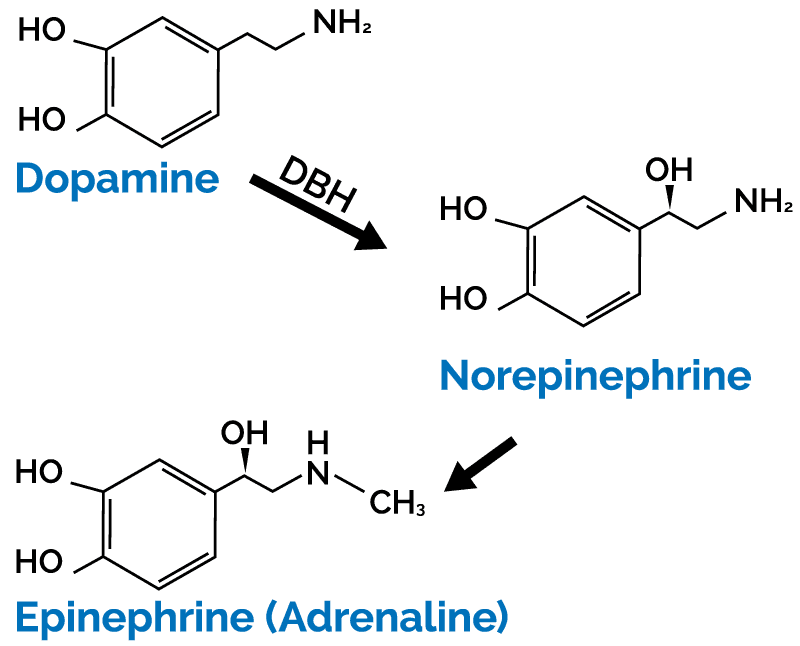
Dopamine beta hydroxylase (DBH) is an enzyme crucial to the balance of dopamine and norepinephrine in the body. It is required for the conversion of dopamine into norepinephrine. DBH is like a lock associated with tyrosine and tyrosine derivatives fit like keys in the lock. Dopamine, a derivative of tyrosine, fits on the DBH enzyme just as a key fits into a lock. DBH then transforms it into norepinephrine when it is functioning adequately, leaving us with proper levels of both dopamine and norepinephrine in the system. When DBH is functioning properly, we experience balanced moods and adequate energy levels. When DBH dysfunction occurs, a wide range of abnormalities can develop.
THE EFFECTS OF DBH DYSFUNCTION
During times of DBH dysfunction, no matter the cause, there will be a decreased conversion of dopamine to norepinephrine. This causes a subsequent rise in dopamine levels and decrease in norepinephrine. Symptoms associated with elevated dopamine include anxiety, depression, fatigue, low blood pressure, sluggish deep tendon reflexes, ptosis, hypotonic muscles, hyperflexible reflexes, weakened facial muscles, and increased stress. Conditions associated with DBH dysfunction are wide ranging. Mental health disorders such as depression, anxiety, bipolar disorder, and schizophrenia have been documented to be associated with dysfunction in the DBH enzyme. Other conditions include postural orthostatic hypotension (POTS), exercise intolerance, fainting, autism spectrum disorder, ADHD, seizures, neurologic conditions, and alcoholism.
CAUSES OF DBH DYSFUNCTION

There are various reasons why DBH function can be altered. These include elevations in clostridia toxins, cofactor deficiency, and genetic single nucleotide polymorphisms (SNPS). When clostridia toxins are elevated, dysfunction of DBH ensues. The toxins produced by this species of bacteria are tyrosine derivatives. Clostridia, when given the opportunity to overgrow, takes tyrosine to produce its toxic metabolites. The most common of these toxins are 4-cresol and HPHPA. These toxins are the tightest binding toxins clostridia produces, although the other tyrosine metabolites of clostridia cause dysfunction too. Since these toxins are produced using tyrosine, they look very similar to dopamine and take its place on the DBH enzyme. This is an irreversible binding that occurs and inhibits DBH function. An Organic Acids Test (OAT) will assess whether a patient has elevated clostridia metabolites and will also assess neurotransmitter status.
When DBH cofactors are deficient, the enzyme does not have the necessary nutrients to efficiently do its job. Cofactors for the DBH enzyme are vitamin C and copper. It has been shown that vitamin C and copper levels can be decreased in patients with neurological disorders. In some populations, individuals may be at a high risk of not getting an adequate amount of these nutrients, like in those with eating disorders or patients that do not have adequate micronutrient nutrition. Also, there are those that have arsenic toxicity. This has been correlated with decreased vitamin C and copper. A heavy metals test should be conducted to rule out this possibility. Supplementation with vitamin C should commence but keep in mind if the person also has elevated oxalates, high doses of vitamin C can potentiate their production. For copper, check their levels using the Copper + Zinc Profile. This information will guide you on dosing copper in respect to zinc.
If clostridia toxins are not elevated, and cofactors are at normal levels, there is always the possibility of someone having a genetic predisposition to decreased enzyme function. There are various SNPs that can impact the function of this enzyme. In these individuals, the symptoms of fatigue, low blood pressure, exercise intolerance, bed wetting, etc. would be expected to be present most of their life or a family history of these symptoms. Genetic testing would give you clues into which SNPs may be playing a role here. Unfortunately, this does not give insight to the functionality of the enzyme. GPL’s Dopamine Beta Hydroxylase Test assesses the functionality of the DBH enzyme via a serum sample. It allows the practitioner to understand how the patient’s genetics is phenotypically affecting them biochemically. The beauty of this test is that even if a person has an inhibition via clostridia toxin, there is no interference and it can be done with accuracy. Practitioners can get a clear understanding of the enzyme’s function and the patient’s ability to adequately convert dopamine to norepinephrine.
TREATMENT FOR DBH DYSFUNCTION
Finding out whether the patient has elevated clostridia metabolites should be done first via Organic Acids Test (OAT) or Microbial Organic Acids Test (MOAT). If they do have elevated clostridia toxins (4-cresol or HPHPA), those should be treated, then re-evaluated for treatment efficacy. This may improve or resolve DBH function and thus any symptoms they have been experiencing due to the clostridia toxins’ inhibition of DBH.
In individuals with decreased DBH functionality found with the Dopamine Beta Hydroxylase Test, there is treatment available. Currently on the market there is a prescription medication, called Droxidopa. Droxidopa is a synthetic amino acid that is converted to norepinephrine by dopamine decarboxylase. This takes the place of the norepinephrine that would have been made via DBH.
In summary, if a patient is having the aforementioned symptoms of DBH dysfunction, various GPL tests are warranted: The first test to screen for DBH dysfunction is the Organic Acids Test (OAT). The markers in the neurotransmitter section will give insight. The HVA, VMA, and HVA/VMA ratios are important. In DBH dysfunction, HVA will be elevated, VMA will be proportionally decreased, and the HVA/VMA ratio will be elevated. This is showing that dopamine is not adequately being converted and there is something blocking the DBH enzyme. Also on the OAT, the clostridia markers (4-cresol and HPHPA) should be assessed to rule in or out its involvement. From here, consider running the Dopamine Beta Hydroxylase Test, a heavy metals test, and the Copper + Zinc Profile for further understanding of a patient’s DBH functionality.






References
- Beuger, M., van Kammen, D. P., Kelley, M. E., & Yao, J. (1996, July). Dopamine turnover in schizophrenia before and after haloperidol withdrawal. CSF, plasma, and urine studies. Retrieved 4AD, from https://www.ncbi.nlm.nih.gov/pubmed/8797194
- Cross, A. J., Crow, T. J., Perry, E. K., Perry, R. H., Blessed, G., & Tomlinson, B. E. (1981). Reduced dopamine-beta-hydroxylase activity in Alzheimers disease. Bmj, 282(6258), 93–94. doi: 10.1136/bmj.282.6258.93
- Garland, E. M. (2019, April 25). Dopamine Beta-Hydroxylase Deficiency. Retrieved April 30, 2020, from https://www.ncbi.nlm.nih.gov/books/NBK1474/
- Kaufmann, H., Norcliffe-Kaufmann, L., & Palma, J.-A. (2015). Droxidopa in neurogenic orthostatic hypotension. Retrieved April 30, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4509799/
- Rahman, M. K., Rahman, F., Rahman, T., & Kato, T. (2009, December). Dopamine-β-Hydroxylase (DBH), Its Cofactors and Other Biochemical Parameters in the Serum of Neurological Patients in Bangladesh. Retrieved 28, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614808/
- Shaw, W. (2010, June). Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Retrieved April 28, 2020, from https://www.ncbi.nlm.nih.gov/pubmed/20423563