When it comes to mycotoxin binding research, we must keep in mind the toxic nature of these toxins. Their toxicity makes the research limited to animals, animal feed, and in vitro studies. Due to the unethical nature of purposefully exposing humans to mycotoxins, there is limited research on these binders in humans. This is acceptable as the binding capacity can still be evaluated efficiently. In your journey to understanding how our bodies process mycotoxins, it is crucial to understand their metabolism. Learn more about the metabolism of the mycotoxins analyzed by Mosaic Diagnostics: Mycotoxin Properties and Metabolism, Part 1 and Part 2.
Binding agents are an integral part of mycotoxin illness detoxification. Trying to decide which binder is correct for which mycotoxin can be a difficult process. This blog provides a collection of research connecting mycotoxins to a good binder choice.
After a positive Organic Acids Test and MycoTOX Profile®, the presence of mold and mycotoxins are usually significant answers for many symptomatic clients. When working with mycotoxicosis, choosing the correct binder can present a challenge. Oftentimes I get the question: which binder is correct for which toxin? Since the research for the binding capacity of each binder isn’t as heavily researched as other agents, it can be daunting to sift through the information. Below is a collection of research connecting mycotoxins to a good binder choice.
In our bodies, toxins are detoxed and excreted through a few pathways. Routes of elimination include urine, stool, bile, and through our skin. Other routes include tears and saliva but are negligible in the realm of detoxification. Another route is breast milk. Since breast milk is a route of excretion this means toxins can be transferred to another life this way. This fact makes binders even that much more crucial in childbearing-age women.
When it comes to binders, bile, and stool are the target routes of elimination. Fat-soluble substances such as dietary lipids, certain vitamins, and fat-soluble toxins like mycotoxins get packaged into bile for absorption and detoxification. During bile’s lifecycle, it gets excreted into the GI tract and is what gives stool its brown color. In the colon, most of the bile is reabsorbed so the liver and gallbladder do not have to work as hard to make more bile. It is recycled and reused. Dysfunction of this phenomenon, bile acid malabsorption, and chronic diarrhea are the main symptoms. Since bile is reabsorbed, in the ileum and jejunum, if toxins are packaged in the bile then the toxins can be reabsorbed as well. They would then re-enter circulation via the hepatic portal system. This is where binders come in handy. Binders will adhere to the bile that packages the toxins and then it cannot be reabsorbed. Due to the nature of this adherence, it cannot be trusted that the bond is irreversible. The lack of a tight bond allows for the bile to be released if not excreted regularly. This means that irregular bowel movements from constipation, lack of fiber, or motility issues could cause the resorption of toxins even with binder usage. Binders by nature are constipating and this should be mitigated and assessed regularly during binder usage. Properly moving bowels through diet and supplements should be achieved before adding any binding agent.

Cholestyramine / Welchol
In the world of prescriptions, cholestyramine and colesevelam hydrochloride, more commonly known as Welchol, are often binders of choice. They are known for their intended use and design, which is their lipid lower activity and use in glycemic control in patients with type 2 diabetes mellitus. They work by directly binding bile in the GI tract. This causes a reduced bile resorption and an increased conversion of cholesterol to bile, via 7a-hydroxylation, thus lowering cholesterol levels. In this process, toxin-laden bile is bound and thus excreted via stool.
These two binders are often used in mycotoxin detoxification protocols. As seen above, this is for good reason. The mycotoxin that responds best to these prescriptions is Ochratoxin A (OTA), according to the research. This is a notorious mycotoxin. OTA is produced by many species of Aspergillus and Penicillium molds. These are two of the most ubiquitous molds in the environment. This fact makes OTA the most common mycotoxin. It is so common that even regular ingestion of commonly moldy foods will most likely expose you to small, negligible amounts of OTA – we outline this in our article: Mycotoxins in Food. In cases of water damage building exposure, these drugs are valuable assets for binding this mycotoxin. Cholestyramine has been shown to effectively bind Aflatoxins and Zearalenone as well.

Charcoal
A few years ago, activated charcoal was popping up in toothpaste, drinks, snacks, and even ice cream. Due to this most people are now familiar with this binder. It is commonly used for firming loose stools, binding toxins from food poisoning, and now for mycotoxin binding. According to the research, most binders will bind to almost anything, including nutrients necessary for life. They are non-discriminating. This fact made the charcoal trend rather troubling for those engaging in high intake of this substance with no regard to its potential danger if not taken responsibly. But, due to this, activated charcoal is an effective toxin binder to just about any toxin that is excreted in the gut. It works similarly to cholestyramine by adsorbing toxins packaged in bile.
Research shows that in foodstuffs and the body activated charcoal is beneficial in mycotoxin binding. ZEA and OTA are bound effectively by charcoal products. This is a good alternative for non-prescribing practitioners. Charcoal has also shown efficacy in adsorbing macrocyclic Trichothecenes. Two of the most common are Verrucarin A and Roridin E, and these are assessed on the MycoTOX Profile®. Other subvarieties of Verrucarin, including ‘J’ have shown binding efficacy with charcoal administration. Also, T-2 toxins from Fusarium are bound by charcoal.
Clays
Another common binding agent is bentonite clay and zeolite clay. These two clays have been touted as working wonders in the cosmetic arena by pulling toxins from the skin. These clays have been shown to bind greatly to toxins in animal feed, reducing the toxic load before consumption. Another clay is montmorillonite clay, also known as Novasil. This clay has ample research as a mycotoxin adsorbent in animal feed, a highly mycotoxin-contaminated source. Great news, these clays do the same adsorbing action in the GI tract. Both agents show great affinity for binding aflatoxins. Also clays have evidence that they can effectively bind OTA, Zearalenone and Enniatin B.
Glucomannan
This water-soluble polysaccharide is a well-touted weight-loss solution. It comes from the elephant yam, konjac. It is a hemicellulose fiber with beta-D-glucose and beta-D-mannose with acetyl groups with beta 1–4 linkages. Due to the lack of enzymes in human saliva to break these linkages, glucomannan goes through the GI tract unchanged. This allows for it to bind without absorption. Due to its content, this fiber can adsorb up to 50 times its weight. Glucomannan has shown efficacy in binding various mycotoxins. Aflatoxin and OTA are major toxins affected by this binder. Others include Zearalenone and Toxin T-2, not much efficacy was seen in binding DON-1 (Deoxynivalenol). Since glucomannan is a fiber this may be an option to consider in more constipated clients with ample water intake.
Chlorella
This next binding agent is a common plant-based agent. Chlorella is a type of freshwater algae that is packaged into a tablet, liquid extracts, and powders. It is often touted as a superfood due to its highly nutritious profile. It is high in protein, vitamins A, C, and E, and is a great source of fiber. Because of its nutrition, chlorella is known for its wound healing, anti-cancer, anti-aging, and immune-boosting potential. In breastfeeding mother’s chlorella intake increased circulating immunoglobulins in breast milk. This plant is great as a heavy metal binder and as a binder of Aflatoxins. It has even been shown to inhibit Aflatoxin B1-induced liver cancer.
Due to the safety profile of chlorella, it is a great binder for all populations. It is difficult to detox a constipated child or a woman expecting a child and is planning to breastfeed. Since so many other binders bind not only toxins but also nutrients it can be difficult to support detox. Adding in small doses of chlorella is a safe and effective way to add in supportive detox without stimulating too much toxin release to the unborn fetus or breastfeeding child.
Humic Acid
Humic acid and its related counterpart, fulvic acid, are the final products of the decomposition of organic matter. They are formed through the humification of plant and animal matter via the biological processes of microorganisms. This byproduct acts as an adsorbent in soil to bind to toxic substances. Agriculturalists use these substances as soil additives to boost the growth and health of their crops due to their concentrated amount of nutrients. Due to its rapid lifecycle, humic acid doesn’t compete for nutrients with the plant or any other organism that uses it. This makes this biotoxin binder simpler to utilize when taking a variety of nutritional supplements.
Not only is humic acid a great biotoxin binder, but it also has shown efficacy as an anti-inflammatory. It also has shown promise in stimulating apoptosis in promyelocytic leukemia cells. This substance, along with fulvic acid, is a wonderful well-rounded addition to any detoxification protocol.

Fiber, Okra and Other Vegetables
Speaking of fiber, in general, fibrous supplements and foods can act as simple overall binders. Fiber from oats, wheat bran, alfalfa, lignans in flax and chia, guar gum, etc have been used as early interventions in lowering cholesterol. It has the same effect that Cholestyramine has on cholesterol. It is due to the bile sequestering activity of these fibers that work to lower cholesterol. In turn, this will also lower the toxic load. Even though fiber doesn’t have much direct research on the binding of specific mycotoxins, it is always a good dietary change to implement. Barley and oats showed the highest absorptive capacity among other fibers when tested. Another great fiber binder to consider is modified citrus pectin (MCP). This binder has shown great efficacy in binding heavy metals such as lead. Though this isn’t a mycotoxin, this shows us that MCP has a great potential utility in any detox protocol.
The first binding agent is a well-known property of certain vegetables. The mucilaginous nature of foods like okra is what gives them their binding power. A mucilage is a sticky, slimy, vicious type of glue that is polysaccharide rich. It is often activated when wet. Think of the gel that is produced when you soak flaxseeds in water5. This gelatinous, gluey nature makes okra and other vegetables able to bind bile acids in the gastrointestinal tract that are carrying toxins. Okra, when studied, had the highest binding capacity to bile and was followed by beets, asparagus, and eggplant. Turnips, green beans, carrots, and cauliflower had less binding capacity than the others, but they were equal to each other. The binding capacity was measured in relation to the capacity of Cholestyramine.
Okra was shown to bind at 15 percent of the capacity of Cholestyramine12. Though this may seem small, this type of binding capacity is from food. With this information, we have more science to back up the benefits of eating certain plant foods as regular staples in your diet. Supplements can be used as well. Since there is a lower binding capacity and most of these foods are fibrous this could be a good option for our constipated clients when it comes to mycotoxin binding.

Peach Stone
This next adsorbent option is one I had not initially thought about. It’s peach stone. The pit of a peach has a composition of biological polymers like cellulose, lignin, and hemicellulose. These compounds increase the intensity of the hydroxyl and phenol groups which give it the adsorbing quality6. Additionally, the substantial structure of the peach stone, with all its openings and canals, also makes for a larger surface area for binding sites. By studying both modified and unmodified peach stone (MPS/PS) we know that this binder can bind Aflatoxin B1, Ochratoxin A (OTA), Deoxynivalenol (DON), Zearalenone (ZON), Diacetoxyscirpenol (DAS) and T-2 toxin. Both MPS and PS, close to a physiologic pH of 7.0, had specific affinities to each toxin6. PS had a higher binding capacity for Aflatoxin B1 than MPS, while both had equal binding capacity to Ochratoxin and T-2 toxin. MPS had a higher affinity for DON, ZON, and DAS as compared to PS6. Overall, the MPS higher the most consistent binding affinity and would be a good overall binder for multiple toxin elevations.

Micro Chitosan
Micro chitosan is another commonly used product. It is a polysaccharide from the outer shell of shellfish like crab and shrimp. It is traditionally used in drug and medicine manufacturing13. As of late, it has been studied as a binding agent in poultry and in corn and wheat crops to reduce mycotoxins. One study showed that chitosan binds Fusarium toxins, fumonisins, and DON, in corn and wheat products14. Another revealed in a poultry gastrointestinal model, that chitosan has a wide binding capacity. In this study chitosan bound to, in increasing order, AFB1, FB1, FB2, OTA, AFG2, AFG1, ZEA, and AFB22,4. Another study also linked chitosan as a good adsorbent to trichothecenes11. Not many binders have been linked to trichothecenes, so this is a benefit to clients who are unfortunately exposed to these. As a side note goji berries have been linked with charcoal as a winning pair in binding and decreasing the toxic effects of T2 toxin10. With micro chitosan take caution and avoid use in anyone who has a shellfish allergy.

Probiotics
This section of biotoxin binders may come as a surprise. Probiotics are best known for their activity in repopulating the GI microbiome after antibiotic use, killing pathogenic organisms like C. difficile, and as a support in a whole host of chronic diseases. What many do not realize is that these organisms can also directly bind to mycotoxins. Lactobacillus strains work to directly bind Aflatoxins, especially the B1 variety. The specific strains are L. pentosus and L. beveris. Another promising strain is L. plantarum C88. This strain works not only to bind to Aflatoxins but also by upregulating the antioxidant activity of glutathione s-transferase. It also shows great binding capacity to the common mycotoxin, Sterigmatocystin.
Strains of saccharomyces also work well to bind mycotoxins. S. cerevisiae has been shown to bind tightly to aflatoxins. It has also shown great efficacy in binding to OTA and Zearalenone. Saccharomyces boulardii, based on clinical observation, has shown efficacy against Gliotoxin. It has also shown efficacy in reducing Aspergillus and Fusarium molds in the GI tract. This will indirectly reduce mycotoxins, as it reduces the producers of mycotoxins including Zearalenone, Enniatin B, OTA, Gliotoxin, and Aflatoxins. Using a variety of these strains will round out not only your gut treatment but also the detoxification process.
Sweeping the functional medicine community is the use of spore or soil-based probiotics. Their efficacy in diversifying the gut microbiome is well documented. This type of probiotic has also been studied as a mycotoxin binder. B. subitillus has been documented to degrade Aflatoxin B1 by 76%, Zearalenone by 84%, and Deoxynivalenol by 78%. Along with other bacillus species (B. lincheniformis, B. megaterium, B. cereus, and B. thuringiensis) these spore base probiotics are efficacious in binding mycotoxins with the addition of manganese9. The Mn2+ is proposed to work with the B. subitillus organism to degrade mycotoxins through manganese peroxidase. Another species, B. coagulans, has been shown to inhibit the production of DON from Fusarium mold1. Other bacillus species have been shown to bind and inactivate other mycotoxins like T-2 and Patulin8. T-2 is also bound by Lactobacillus and Saccharomyces species7. This information shows us that a variety of probiotics are useful in the supportive care of those with mycotoxin exposure.
All in all, binding agents are an integral part of mycotoxin illness detoxification. Whether someone has 1 or 10 mycotoxins populate on the MycoTOX Profile, having the correct binders can be a challenge. Hopefully, this can be used as a resource to guide you in planning toxin binder regimens to best help your clients. Using a combination of binding agents will allow for well-rounded binding capacity in any mycotoxin toxicity case.
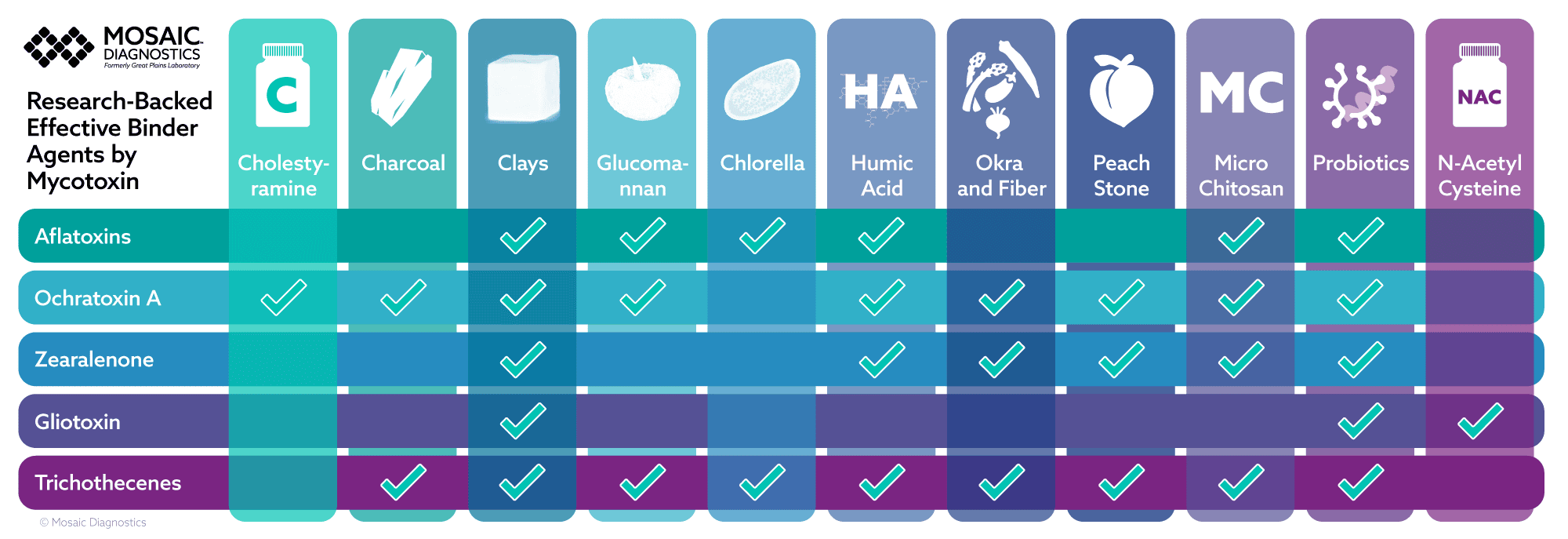
Printable Chart: Binders by Mycotoxin
Click the chart to print. This is a table matching mycotoxin with binders that have research for their binding affinity:
References
- A. C. K. K. (n.d.). Antifungal activity of bacillus coagulans against Fusarium SP. Acta microbiologica Polonica. https://pubmed.ncbi.nlm.nih.gov/12588102/
- Abbasi Pirouz, A., Selamat, J., Zafar Iqbal, S., & Iskandar Putra Samsudin, N. (2020, February 12). Efficient and simultaneous chitosan-mediated removal of 11 mycotoxins from Palm Kernel Cake. MDPI. https://www.mdpi.com/2072-6651/12/2/115
- Adhikari, M., Negi, B., Kaushik, N., Adhikari, A., Al-Khedhairy, A. A., Kaushik, N. K., & Choi, E. H. (2017, May 16). T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5464924/
- Delgado-Cedeño, A., Hernández-Martínez, S. P., Ramos-Zayas, Y., Marroquín-Cardona, A. G., Méndez-Zamora, G., Franco-Molina, M. A., & Kawas, J. R. (2022, November 10). Insoluble chitosan complex as a potential adsorbent for aflatoxin B1 in Poultry Feed. Frontiers. https://www.frontiersin.org/articles/10.3389/fmats.2022.1044495/full
- A grammatical dictionary of botanical latin. MO Garden main page. (n.d.). http://www.mobot.org/mobot/latindict/keyDetail.aspx?keyWord=mucilaginous
- In vitro evaluation of the efficacy of Peach Stones as … – doiserbia. (n.d.). http://www.doiserbia.nb.rs/img/doi/0352-4906/2013/0352-49061324287L.pdf
- Janik, E., Niemcewicz, M., Podogrocki, M., Ceremuga, M., Stela, M., & Bijak, M. (2021, November 14). T-2 toxin-the most toxic trichothecene mycotoxin: Metabolism, toxicity, and decontamination strategies. MDPI. https://www.mdpi.com/1420-3049/26/22/6868
- Piotrowska, M. (2021, November 19). Microbiological decontamination of mycotoxins: Opportunities and limitations. MDPI. https://www.mdpi.com/2072-6651/13/11/819
- Qin, X., Su, X., Tu, T., Zhang, J., Wang, X., Wang, Y., Wang, Y., Bai, Y., Yao, B., Luo, H., & Huang, H. (2021). Enzymatic degradation of multiple major mycotoxins by dye-decolorizing peroxidase from bacillus subtilis. Toxins, 13(6), 429. https://doi.org/10.3390/toxins13060429
- Scientific Research Publishing. (2014, December 1). Evaluation of gojiextract and charcoal as antioxidant on T-2 toxin administration onliver male mice. Food and Nutrition Sciences. https://www.scirp.org/html/3-2701332_52009.html
- Solís-Cruz, B., Hernández-Patlán, D., Beyssac, E., Latorre, J. D., Hernandez-Velasco, X., Merino-Guzman, R., Tellez, G., & López-Arellano, R. (2017, October 19). Evaluation of chitosan and cellulosic polymers as binding adsorbent materials to prevent aflatoxin B1, Fumonisin B1, ochratoxin, Trichothecene, deoxynivalenol, and Zearalenone Mycotoxicoses through an in vitro gastrointestinal model for poultry. Polymers. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6418884/
- Trowell, H. C., Suckling, K. E., Story, J. A., Rossi, S. S., Kritchevsky, D., Kahlon, T. S., Hofmann, A. F., Hecht, S. S., Gautam, M., Eastwood, M. A., Diwanay, S., Daggy, B. P., Carey, M. C., Balmer, J., Ames, B. N., & Anderson, J. W. (2006, October 6). In vitro binding of bile acids by okra, beets, asparagus, eggplant, turnips, green beans, carrots, and cauliflower. Food Chemistry.
- WebMD. (n.d.). Chitosan: Overview, uses, side effects, precautions, interactions, dosing and reviews. WebMD. https://www.webmd.com/vitamins/ai/ingredientmono-625/chitosan
- Zachetti VGL;Cendoya E;Nichea MJ;Chulze SN;Ramirez ML; (n.d.). Preliminary study on the use of Chitosan as an eco-friendly alternative to control fusarium growth and mycotoxin production on maize and wheat. Pathogens (Basel, Switzerland). https://pubmed.ncbi.nlm.nih.gov/30841490/
- Agawane, S. B., and P. Lonkar. “Effect of Probiotic Containing Saccharomyces Boulardii on Agawane, S. B., and P. Lonkar. “Effect of Probiotic Containing Saccharomyces Boulardii on Experimental Ochratoxicosis in Broilers: Hematobiochemical Studies.: Semantic Scholar.” Undefined, 1 Jan. 1970, SOURCE.
- Ardeshir Mohaghegh,Mohammad Chamani,Mahmoud Shivazad,Ali Asghar Sadeghi &Nazar Afzali. “Effect of Esterified Glucomannan on Broilers Exposed to Natural Mycotoxin-Contaminated Diets.” Taylor & Francis, SOURCE.
- Armando, M.R., et al. “Adsorption of Ochratoxin A and Zearalenone by Potential Probiotic Saccharomyces Cerevisiae Strains and Its Relation with Cell Wall Thickness.” Journal of Applied Microbiology, vol. 113, no. 2, 2012, pp. 256–264., doi:10.1111/j.1365-2672.2012.05331.x.
- “Chlorophyll and Chlorophyllin.” Linus Pauling Institute, 1 Jan. 2021, SOURCE.
- D. Lloyd-Jones, R. Adams, et al. “Impact of Daily Chlorella Consumption on Serum Lipid and Carotenoid Profiles in Mildly Hypercholesterolemic Adults: a Double-Blinded, Randomized, Placebo-Controlled Study.” Nutrition Journal, BioMed Central, 1 Jan. 1970, SOURCE.
- De Mil, Thomas, et al. “Characterization of 27 Mycotoxin Binders and the Relation with in Vitro Zearalenone Adsorption at a Single Concentration.” Toxins, MDPI, 5 Jan. 2015, SOURCE.
- Devreese M;Girgis GN;Tran ST;De Baere S;De Backer P;Croubels S;Smith TK; “The Effects of Feed-Borne Fusarium Mycotoxins and Glucomannan in Turkey Poults Based on Specific and Non-Specific Parameters.” Food and Chemical Toxicology: an International Journal Published for the British Industrial Biological Research Association, U.S. National Library of Medicine, SOURCE.
- El Khoury, Rhoda, et al. “OTA Prevention and Detoxification by Actinobacterial Strains and Activated Carbon Fibers: Preliminary Results.” MDPI, Multidisciplinary Digital Publishing Institute, 24 Mar. 2018, SOURCE.
- Garcia Diaz, Tatiana, et al. “Use of Live Yeast and Mannan-Oligosaccharides in Grain-Based Diets for Cattle: Ruminal Parameters, Nutrient Digestibility, and Inflammatory Response.” PloS One, Public Library of Science, 14 Nov. 2018, SOURCE.
- Guo M;Hou Q;Waterhouse GIN;Hou J;Ai S;Li X; “A Simple Aptamer-Based Fluorescent Aflatoxin B1 Sensor Using Humic Acid as Quencher.” Talanta, U.S. National Library of Medicine, SOURCE.
- Hamidi, Adel, et al. “The Aflatoxin B1 Isolating Potential of Two Lactic Acid Bacteria.” Asian Pacific Journal of Tropical Biomedicine, vol. 3, no. 9, 2013, pp. 732–736., doi:10.1016/s2221-1691(13)60147-1.
- Hope, Janette. “A Review of the Mechanism of Injury and Treatment Approaches for Illness Resulting from Exposure to Water-Damaged Buildings, Mold, and Mycotoxins.” The Scientific World Journal, Hindawi, 18 Apr. 2013, SOURCE.
- J;, Santos RR;Vermeulen S;Haritova A;Fink-Gremmels. “Isotherm Modeling of Organic Activated Bentonite and Humic Acid Polymer Used as Mycotoxin Adsorbents.” Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, U.S. National Library of Medicine, SOURCE.
- Jay Y. Jacela, DVM; Joel M. DeRouchey, PhD; Mike D. Tokach, PhD; Robert D. Goodband, PhD; Jim L. Nelssen, PhD; David G. Renter, DVM, PhD; Steve S. Dritz, DVM, PhD. Fact Sheet: Mold Inhibitors, Mycotoxin Binders, and Antioxidants, SOURCE.
- Jubert, Carole, et al. “Effects of Chlorophyll and Chlorophyllin on Low-Dose Aflatoxin B(1) Pharmacokinetics in Human Volunteers.” Cancer Prevention Research (Philadelphia, Pa.), U.S. National Library of Medicine, Dec. 2009, SOURCE.
- Kerkadi A;Barriault C;Tuchweber B;Frohlich AA;Marquardt RR;Bouchard G;Yousef IM; “Dietary Cholestyramine Reduces Ochratoxin A-Induced Nephrotoxicity in the Rat by Decreasing Plasma Levels and Enhancing Fecal Excretion of the Toxin.” Journal of Toxicology and Environmental Health. Part A, U.S. National Library of Medicine, SOURCE.
- Kraljević Pavelić, Sandra, et al. “Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in Vivo.” Frontiers in Pharmacology, Frontiers Media S.A., 27 Nov. 2018, SOURCE.
- Kumar, C. B. ; Reddy, B. S. V. ; Gloridoss, R. G. ; Prabhu, T. M. ; Suresh, B. N. “ Effect of MOS Based Toxin Binder on Low Level Citrinin Toxicity in Commercial Broilers.” Mysore Journal of Agricultural Sciences, vol. 48, no. 1, 2014, pp. 75–82.
- L,Haus M;Žatko D;Vašková J;Vaško. “The Effect of Humic Acid in Chronic Deoxynivalenol Intoxication.” Environmental Science and Pollution Research International, U.S. National Library of Medicine, SOURCE.
- Lauterburg BH, Dickson ER, Pineda AA, Carlson GL, Taswell HF. “Removal of Bile Acids and Bilirubin by Plasmaperfusion of U.S.P. Charcoal-Coated Glass Beads.” Europe PMC, 30 Sept. 1979, SOURCE.
- Li, Yan, et al. “Research Progress on the Raw and Modified Montmorillonites as Adsorbents for Mycotoxins: A Review.” Applied Clay Science, Elsevier, 30 July 2018, SOURCE.
- Naumann, Susanne, et al. “In Vitro Interactions of Dietary Fibre Enriched Food Ingredients with Primary and Secondary Bile Acids.” Nutrients, vol. 11, no. 6, 2019, p. 1424., doi:10.3390/nu11061424. 23. Riaz, Sana. “Cholestyramine Resin.” StatPearls [Internet]., U.S. National Library of Medicine, 25 May 2020, SOURCE.
- Rotter, R G, et al. “Influence of Dietary Charcoal on Ochratoxin A Toxicity in Leghorn Chicks.” Canadian Journal of Veterinary Research = Revue Canadienne De Recherche Veterinaire, U.S. National Library of Medicine, Oct. 1989, SOURCE.
- Vahouny, George V., et al. “Dietary Fibers: V. Binding of Bile Salts, Phospholipids and Cholesterol from Mixed Micelles by Bile Acid Sequestrants and Dietary Fibers.” Lipids, vol. 15, no. 12, 1980, pp. 1012–1018., doi:10.1007/bf02534316. 26. “Verrucarin A (T3D3720).” T3DB, SOURCE.
- S,Baker; W,Shaw. “Case Study: Rapid Complete Recovery From An Autism Spectrum Disorder After Treatment of Aspergillus With The Antifungal Drugs Itraconazole And Sporanox.” Integrative Medicine (Encinitas, Calif.), U.S. National Library of Medicine, SOURCE.
- Wang JS;Luo H;Billam M;Wang Z;Guan H;Tang L;Goldston T;Afriyie-Gyawu E;Lovett C;Griswold J;Brattin B;Taylor RJ;Huebner HJ;Phillips TD; “Short-Term Safety Evaluation of Processed Calcium Montmorillonite Clay (NovaSil) in Humans.” Food Additives and Contaminants, U.S. National Library of Medicine, SOURCE.
- Yang, Hsin-Ling, et al. “Humic Acid Induces Apoptosis in Human Premyelocytic Leukemia HL-60 Cells.” Life Sciences, Pergamon, 25 June 2004, SOURCE.
- Zhao ZY;Liang L;Fan X;Yu Z;Hotchkiss AT;Wilk BJ;Eliaz I; “The Role of Modified Citrus Pectin as an Effective Chelator of Lead in Children Hospitalized with Toxic Lead Levels.” Alternative Therapies in Health and Medicine, U.S. National Library of Medicine, SOURCE.
- https://pubmed.ncbi.nlm.nih.gov/35158659/