The liver is a vital organ responsible for numerous essential metabolic and physiological functions, including detoxification. This process involves the elimination of cellular debris, medications, environmental toxicants, and mycotoxins through a series of well-coordinated phases: Phase I, Phase II, and Phase III. The primary role of liver detoxification is to transform lipophilic substances into more hydrophilic forms to facilitate their elimination from the body, as covered in The Liver: It’s Important Role in Detoxification blog. Substances such as those detected on our TOXDetect Profile® for environmental toxicants and our MycoTOX Profile™ for mycotoxins range in their lipophilicity and undergo this transformation.
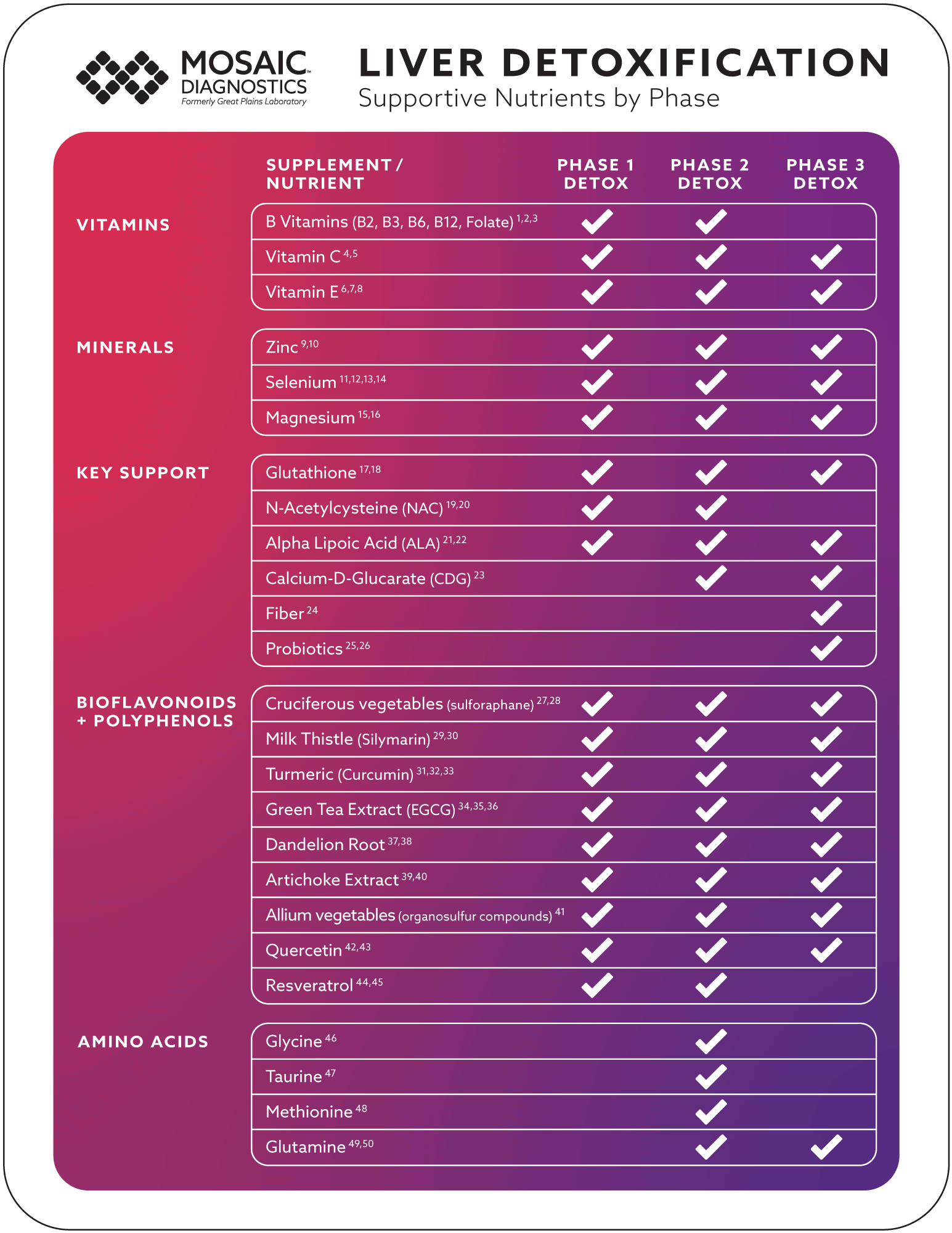
This review explores various nutrients and plant derivatives that support the biochemical reactions facilitated by specific enzymes in each detoxification phase, promoting overall health of not just the liver, but the whole body as well. You will find an easy-to-follow table categorizing the nutrients into Vitamins, Minerals, Key Liver Support Nutrients, Bioflavonoids & Polyphenols, and Amino Acids with detailed explanations in the following sections.
Key Supportive Nutrients of Liver Detoxification
When considering supplements and nutrients that support the intrinsic processes of liver detoxification, it is evident that many of these nutrients often have multiple roles rather than a singular function. They contribute to various aspects of liver detoxification – including enhancing the liver’s enzymatic reactions, protecting against oxidative stress due to the generation of reactive oxygen species, promoting efficient excretion of toxic substances, and supporting liver cell health and regeneration.
Keep learning about liver detoxification with our complementary “Phases of Liver Detoxification” chart.
Vitamins
B-Vitamins
B vitamins, particularly B2 (riboflavin), B3 (niacin), B6 (pyridoxine), B9 (folate), and B12 (cobalamin), are essential cofactors for the cytochrome P450 enzymes.51
Vitamin B6 helps in the conversion of homocysteine to cysteine (a precursor for glutathione synthesis) and been shown to protect hepatocytes from oxidative damage by reducing lipid peroxidation, protein oxidation, and DNA damage.52
Vitamin B12 (cobalamin) and folate (vitamin B9) are critical for the synthesis of S-adenosylmethionine (SAMe). SAMe is a key methyl donor in numerous methylation reactions that take place in phase II and supports glutathione levels.53
Vitamin C
Vitamin C (ascorbic acid) is a potent antioxidant that neutralizes reactive oxygen species (ROS) and oxidative stress in hepatocytes. Some evidence suggests that vitamin C supplementation can lower markers of oxidative stress, such as malondialdehyde (MDA), and increase antioxidant enzyme activities, including superoxide dismutase (SOD) and catalase (CAT).54 55
Vitamin C supports phase II by maintaining adequate levels of glutathione, which is critical for maintaining conjugation reactions.56 It may also exert inflammation-modulation effects by modulating cytokine production and mitigating hepatic injury.57
Vitamin E
Vitamin E, particularly in the form of α-tocopherol, also neutralizes ROS and helps to prevent oxidative damage to hepatocytes.58 In phase II detoxification, vitamin E influences the activity of glutathione-dependent enzymes, such as glutathione-S-transferases (GSTs).59 Lastly, vitamin E can mitigate potential liver damage induced by various xenobiotics, such as carbon tetrachloride and organophosphate pesticides, due to its ability to reduce lipid peroxidation and maintain cellular integrity.60
Minerals
Essential minerals such as magnesium, zinc, and selenium are important for the proper functioning of detoxification enzymes by serving as cofactors for the different reactions in the various phases of liver detoxification.61
Zinc
Zinc modulates the activity of cytochrome p450 enzymes, particularly CYP2E1, which is involved in phase I detoxification. In phase II, it enhances the activity of glutathione-related enzymes and increases hepatic glutathione levels.62 Zinc supports phase III by upregulating metallothionein (MT) and Nrf2 pathways. MT is crucial for binding and sequestering heavy metals while Nrf2 regulates the expression of antioxidant proteins that protect against oxidative damage.63
Zinc is also beneficial for stabilizing cellular membranes and DNA and protects hepatocytes from apoptosis and genotoxicity induced by toxicants like aflatoxin B1.64
Selenium
Selenium is a crucial component of selenoproteins, such as glutathione peroxidase (GPx), which play a significant role in neutralizing ROS and supplementing selenium has been shown to increase GPx activity.65 66 Selenium may also induce phase II enzymes like GSTs and modulate the Nrf2 pathway for phase III.67 68
Another property of selenium is its ability to mitigate liver damage induced by heavy metals like cadmium and mercury.69
Magnesium
Adequate magnesium helps ensure that cytochrome p450 enzymes function optimally by maintaining cellular energy metabolism and mitochondrial function.70 In phase II, magnesium enhances the activity of enzymes such as glutathione-S-transferases (GSTs). Furthermore, magnesium supplementation has been shown to modulate inflammatory signaling pathways and prevent hepatocyte damage.71
Key Liver Support Nutrients
Glutathione
Glutathione (GSH) is a critical intracellular antioxidant that directly scavenges ROS and reactive nitrogen species (RNS), therefore protecting hepatocytes from oxidative stress.72 In phase II, GSH plays a pivotal role by conjugating with xenobiotics and endogenous metabolites through the action of GSTs.73 Lastly, GSH modulates the Nrf2 pathway and enhances upregulation of genes involved in GSH synthesis and utilization.74
N-acetyl cysteine (NAC)
N-acetyl cysteine (NAC) is a precursor to GSH, a critical antioxidant that helps neutralize reactive intermediates and facilitate their excretion.75 Beyond supporting GSH levels and functions, NAC also directly scavenges ROS and enhances the antioxidant enzymes such as GPx and catalase (CAT).76 Additionally, sulfur donors like NAC, organosulfur in allium vegetables, and sulforaphane from cruciferous vegetables have been shown to induce phase II enzymes.77
Alpha-lipoic Acid (ALA)
Alpha-lipoic acid (ALA) serves as both a water- and fat-soluble antioxidant, and evidence suggests that supplementation may support the regeneration of other antioxidants like vitamin C and vitamin E – thereby enhancing the liver’s defense against oxidative damage.78 ALA activates Nrf2 which, in turn, up-regulates the expression of various antioxidant and detoxification enzymes and may also mitigate liver damage induced by hepatotoxic agents, including carbon tetrachloride and acetaminophen.79,80
Calcium-D-Glucarate (CDG)/D-Glucaric Acid
D-glucaric acid, along with its salts (including calcium-d-glucarate), supports liver detoxification by reducing ROS production, inhibiting β-glucuronidase, and may have evidence of downregulating hepatocyte apoptosis.81
Bioflavonoids and Polyphenols
Sulforaphane
Sulforaphane, found in cruciferous vegetables like broccoli, is a potent inducer of GSTs in phase II detoxification and a Nrf2 activator.82 Some evidence suggests sulforaphane has been shown to induce the expression of multidrug resistance associated proteins (MRPs), the key efflux transporters in the liver.83
Silymarin
Milk thistle (Silybum marianum) is widely recognized for its active component, silymarin, which has antioxidant properties and may support healthy inflammatory function.84 Compounds such as silymarin, curcumin, EGCG, and quercetin have been shown to modulate the activity of cytochrome P450 enzymes and provide antioxidant protection in phase I detoxification.85 Silymarin supplementation may also protect the liver from damage caused by agents such as acetaminophen and carbon tetrachloride.86
Curcumin
Curcumin, a polyphenol derived from turmeric (Curcuma longa), modulates cytochrome p450 enzymes, particularly CYP1A1, CYP1A2, and CPY3A4, and increases the activity of GSTs and quinone reductase (QR).87,88 Curcumin has additionally been documented to activate Nrf2 and upregulate antioxidant response elements, thereby supporting the expression of phase III transporters.89
EGCG
Epigallocatechin-3-gallate (EGCG) is a polyphenol derived from green tea. Like curcumin, EGCG also modulates the same CYP enzymes in phase I.90 EGCG is a potent inducer of phase II enzymes: GST, GPx, and NAD(P)H quinone oxidoreductase 1 (NQO1).91 It has been shown to activate the Nrf2 pathway, enhancing the expression of detoxification enzymes and efflux transporters.92
Dandelion Root
Dandelion root (Taraxacum officinale) has been shown to increase hepatic antioxidant activities, including catalase, GST, GPx, and GSH reductase.93 It also exerts hepatoprotective effects by having the ability to reduce fibrinous deposits and to support a healthy hepatic inflammatory response.94
Artichoke Extract
Artichoke extract (Cynara scolymus) not only upregulates enzymatic reactions in phase I and phase II detoxification, but it also exhibits choleretic activity and has been shown to increase bile flow and the formation of bile compounds.95,96 This is crucial for the excretion of conjugated toxic substances.
Quercetin
Quercetin is a flavonoid present in many fruits, vegetables, and grains. Quercetin modulates CYP450 enzymes in phase I and is a potent inducer of phase II enzymes (GST and NAD(P)H quinone oxidoreductase-1 (NQO1)) and the Nrf2 pathway in phase III.97,98
Resveratrol
Resveratrol is a natural polyphenol found in fruits that has similar actions to the other bioflavonoids previously mentioned. However, resveratrol uniquely also induces the expression of UDP-glucuronosyltransferase (UGT) enzymes, which are crucial for glucuronidation in phase II.99,100
Amino Acids
Although amino acids such as glycine, taurine, methionine, and glutamine may not have overarching effects on detoxification and hepatoprotection as compared to the other nutrients we’ve covered thus far, their function in supporting liver health is of note.
Glycine is involved in the synthesis of glutathione.101 Taurine, synthesized from methionine and cysteine in the liver, has significant antioxidant properties and plays a role in phase II amino acid conjugation.102 Methionine is a precursor of SAMe and plays a role in transmethylation and transsulfuration pathways in phase II.103 Lastly, glutamine enhances bile acid secretion, plays a role in phase II conjugation, and may also activate the Nrf2 pathway.104,105
Binders for General Detoxification
The final category of supplements and nutrients to keep in mind when enhancing liver detoxification are “binders”. Binders, or binding agents, also aid in removing toxic substances from the body. These substances work by adsorbing toxic substances and metabolic byproducts, preventing their reabsorption into the bloodstream, facilitating their excretion through the digestive tract. This in turn lowers the burden on the liver and kidneys, while also potentially supporting the integrity of the gut barrier.106,107,108 Some of the binders clinicians commonly use include activated charcoal, zeolite or bentonite clay, humic and fulvic acids, chlorella and soluble fiber products like psyllium husk, glucomannan, apple pectin, and modified citrus pectin (MCP).
Summary
The vitamins, minerals, specific ingredients, phytonutrients, and amino acids discussed above are all important in the support of the liver’s detoxification phases. These nutrients and compounds can have numerous roles in the different stages of the detoxification process such as reducing oxidative stress, supporting enzymatic function, and being a part of synthesizing important compounds associated with detoxification. They also can enhance detoxification processes by promoting cellular protection and reducing inflammation. Moreover, the binders assist in removing toxins from the body, with the hopes of reducing the overall load on the system. By incorporating these types of nutrients through diet and supplementation, it can help maintain liver health and support overall detoxification, especially in the presence of a toxic chemicals.

References
- Parke DV. Nutritional requirements for detoxification of environmental chemicals. Food Addit Contam. 1991;8(3):381-396. doi:10.1080/02652039109373987.
- Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chem Biol Interact. 2006;163(1-2):113-132. doi:10.1016/j.cbi.2006.05.010
- Halsted CH. B-Vitamin dependent methionine metabolism and alcoholic liver disease. Clin Chem Lab Med. 2013;51(3):457-465. doi:10.1515/cclm-2012-0308
- Al Garea MH, Alqasoumi AA, Alqahtani SA, Hadadi AH, Emara AM. Vitamin C as a potential ameliorating agent against hepatotoxicity among alcoholic abusers. Eur Rev Med Pharmacol Sci. 2023;27(8):3322-3335. doi:10.26355/eurrev_202304_32103
- Ozturk IC, Ozturk F, Gul M, Ates B, Cetin A. Protective effects of ascorbic acid on hepatotoxicity and oxidative stress caused by carbon tetrachloride in the liver of Wistar rats. Cell Biochem Funct. 2009;27(5):309-315. doi:10.1002/cbf.1575
- Naziroğlu M, Cay M, Ustündağ B, Aksakal M, Yekeler H. Protective effects of vitamin E on carbon tetrachloride-induced liver damage in rats. Cell Biochem Funct. 1999;17(4):253-259. doi:10.1002/(SICI)1099-0844(199912)17:4<253::AID-CBF837>3.0.CO;2-R
- van Haaften RI, Haenen GR, Evelo CT, Bast A. Effect of vitamin E on glutathione-dependent enzymes. Drug Metab Rev. 2003;35(2-3):215-253. doi:10.1081/dmr-120024086
- Sen Gupta P, Karmakar S, Biswas I, et al. Vitamin E alleviates chlorpyrifos induced glutathione depletion, lipid peroxidation and iron accumulation to inhibit ferroptosis in hepatocytes and mitigate toxicity in zebrafish. Chemosphere. 2024;359:142252. doi:10.1016/j.chemosphere.2024.142252
- Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005;26(4-5):391-404. doi:10.1016/j.mam.2005.07.002
- Liu J, Zhou ZX, Zhang W, Bell MW, Waalkes MP. Changes in hepatic gene expression in response to hepatoprotective levels of zinc. Liver Int. 2009;29(8):1222-1229. doi:10.1111/j.1478-3231.2009.02007
- Zhang C, Ge J, Lv M, Zhang Q, Talukder M, Li JL. Selenium prevent cadmium-induced hepatotoxicity through modulation of endoplasmic reticulum-resident selenoproteins and attenuation of endoplasmic reticulum stress. Environ Pollut. 2020;260:113873. doi:10.1016/j.envpol.2019.113873
- Shen Y, Huang H, Wang Y, Yang R, Ke X. Antioxidant effects of Se-glutathione peroxidase in alcoholic liver disease. J Trace Elem Med Biol. 2022;74:127048. doi:10.1016/j.jtemb.2022.127048
- Xiao H, Parkin KL. Induction of phase II enzyme activity by various selenium compounds. Nutr Cancer. 2006;55(2):210-223. doi:10.1207/s15327914nc5502_13
- Indumathi MC, Swetha K, Abhilasha KV, et al. Selenium Ameliorates Acetaminophen-Induced Oxidative Stress via MAPK and Nrf2 Pathways in Mice. Biol Trace Elem Res. 2024;202(6):2598-2615. doi:10.1007/s12011-023-03845-3
- Yang YZ, Liu ZH, Wang SC, et al. Magnesium isoglycyrrhizinate alleviates fructose-induced liver oxidative stress and inflammatory injury through suppressing NOXs. Eur J Pharmacol. 2020;883:173314. doi:10.1016/j.ejphar.2020.173314
- Zhao XJ, Yang YZ, Zheng YJ, et al. Magnesium isoglycyrrhizinate blocks fructose-induced hepatic NF-κB/NLRP3 inflammasome activation and lipid metabolism disorder [published correction appears in Eur J Pharmacol. 2021 Oct 15;909:174440. doi: 10.1016/j.ejphar.2021.174440]. Eur J Pharmacol. 2017;809:141-150. doi:10.1016/j.ejphar.2017.05.032
- Morris G, Anderson G, Dean O, et al. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. 2014;50(3):1059-1084. doi:10.1007/s12035-014-8705-x
- Uchida Y, Ferdousi F, Takahashi S, Isoda H. Comprehensive Transcriptome Profiling of Antioxidant Activities by Glutathione in Human HepG2 Cells. Molecules. 2024;29(5):1090. Published 2024 Feb 29. doi:10.3390/molecules29051090
- Uchida Y, Ferdousi F, Takahashi S, Isoda H. Comprehensive Transcriptome Profiling of Antioxidant Activities by Glutathione in Human HepG2 Cells. Molecules. 2024;29(5):1090. Published 2024 Feb 29. doi:10.3390/molecules29051090
- de Andrade KQ, Moura FA, dos Santos JM, de Araújo OR, de Farias Santos JC, Goulart MO. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. Int J Mol Sci. 2015;16(12):30269-30308. Published 2015 Dec 18. doi:10.3390/ijms161226225
- Jung TS, Kim SK, Shin HJ, Jeon BT, Hahm JR, Roh GS. α-lipoic acid prevents non-alcoholic fatty liver disease in OLETF rats. Liver Int. 2012;32(10):1565-1573. doi:10.1111/j.1478-3231.2012.02857
- Hossain KFB, Akter M, Rahman MM, et al. Amelioration of Metal-Induced Cellular Stress by α-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells. Int J Environ Res Public Health. 2021;18(4):2126. Published 2021 Feb 22. doi:10.3390/ijerph18042126
- Ayyadurai VAS, Deonikar P, Fields C. Mechanistic understanding of D-glucaric acid to support liver detoxification essential to muscle health using a computational systems biology approach. Nutrients. 2023;15(3):733. doi:10.3390/nu15030733.
- Ciocan D, Spatz M, Trainel N, et al. Modulation of the Bile Acid Enterohepatic Cycle by Intestinal Microbiota Alleviates Alcohol Liver Disease. Cells. 2022;11(6):968. Published 2022 Mar 11. doi:10.3390/cells11060968
- Liu Y, Chen K, Li F, et al. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology. 2020;71(6):2050-2066. doi:10.1002/hep.30975.
- Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147(6):1327-1337.e3. doi:10.1053/j.gastro.2014.08.031
- Jayasuriya R, Dhamodharan U, Ali D, Ganesan K, Xu B, Ramkumar KM. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine. 2021;92:153755. doi:10.1016/j.phymed.2021.153755
- Maher JM, Dieter MZ, Aleksunes LM, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46(5):1597-1610. doi:10.1002/hep.21831
- Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144-149. doi:10.4254/wjh.v6.i3.144
- Simón J, Casado-Andrés M, Goikoetxea-Usandizaga N, Serrano-Maciá M, Martínez-Chantar ML. Nutraceutical properties of polyphenols against liver diseases. Nutrients. 2020;12(11). doi:10.3390/nu12113517
- Muhammad I, Wang H, Sun X, et al. Dual Role of Dietary Curcumin Through Attenuating AFB1-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB1 Bioactivation and Detoxification. Front Pharmacol. 2018;9:554. Published 2018 May 25. doi:10.3389/fphar.2018.00554
- Wang H, Muhammad I, Li W, Sun X, Cheng P, Zhang X. Sensitivity of Arbor Acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of phase-II enzymes and Nrf2. Environ Toxicol Pharmacol. 2018;59:94-104. doi:10.1016/j.etap.2018.03.003
- Jayasuriya R, Dhamodharan U, Ali D, Ganesan K, Xu B, Ramkumar KM. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine. 2021;92:153755. doi:10.1016/j.phymed.2021.153755
- Yao HT, Li CC, Chang CH. Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats. Nutrients. 2019;11(8):1862. Published 2019 Aug 10. doi:10.3390/nu11081862
- Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46(4):1271-1278. doi:10.1016/j.fct.2007.10.006
- Wang Y, Wu J, Wang L, et al. Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins (Basel). 2022;14(12):876. Published 2022 Dec 15. doi:10.3390/toxins14120876
- You Y, Yoo S, Yoon HG, et al. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol. 2010;48(6):1632-1637. doi:10.1016/j.fct.2010.03.037
- Domitrović R, Jakovac H, Romić Z, Rahelić D, Tadić Z. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J Ethnopharmacol. 2010;130(3):569-577. doi:10.1016/j.jep.2010.05.046
- Liao GC , Jhuang JH , Yao HT . Artichoke leaf extract supplementation lowers hepatic oxidative stress and inflammation and increases multidrug resistance-associated protein 2 in mice fed a high-fat and high-cholesterol diet [published correction appears in Food Funct. 2021 Sep 20;12(18):8812. doi: 10.1039/d1fo90070f]. Food Funct. 2021;12(16):7239-7249. doi:10.1039/d1fo00861g
- Kirchhoff R, Beckers C, Kirchhoff GM, Trinczek-Gärtner H, Petrowicz O, Reimann HJ. Increase in choleresis by means of artichoke extract. Phytomedicine. 1994;1(2):107-115. doi:10.1016/S0944-7113(11)80027-9
- Chang HS, Ko M, Ishizuka M, et al. Sodium 2-propenyl thiosulfate derived from garlic induces phase II detoxification enzymes in rat hepatoma H4IIE cells. Nutr Res. 2010;30(6):435-440. doi:10.1016/j.nutres.2010.06.007.
- Kim M, Jee SC, Kim KS, Kim HS, Yu KN, Sung JS. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants (Basel). 2021;10(5):787. Published 2021 May 16. doi:10.3390/antiox10050787
- Ji LL, Sheng YC, Zheng ZY, Shi L, Wang ZT. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12-23. doi:10.1016/j.freeradbiomed.2015.03.035
- Chow HH, Garland LL, Hsu CH, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010;3(9):1168-1175. doi:10.1158/1940-6207.CAPR-09-0155
- Chupradit S, Bokov D, Zamanian MY, Heidari M, Hakimizadeh E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam Clin Pharmacol. 2022;36(3):468-485. doi:10.1111/fcp.12746
- Rom O, Liu Y, Liu Z, et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci Transl Med. 2020;12(572):eaaz2841. doi:10.1126/scitranslmed.aaz2841
- Mizota T, Hishiki T, Shinoda M, et al. The hypotaurine-taurine pathway as an antioxidative mechanism in patients with acute liver failure. J Clin Biochem Nutr. 2022;70(1):54-63. doi:10.3164/jcbn.21-50
- Jung YS. Metabolism of Sulfur-Containing Amino Acids in the Liver: A Link between Hepatic Injury and Recovery. Biol Pharm Bull. 2015;38(7):971-974. doi:10.1248/bpb.b15-00244
- Häussinger D, Graf D, Weiergräber OH. Glutamine and cell signaling in liver. J Nutr. 2001;131(9 Suppl):2509S-4S. doi:10.1093/jn/131.9.2509S
- Schemitt EG, Hartmann RM, Colares JR, et al. Protective action of glutamine in rats with severe acute liver failure. World J Hepatol. 2019;11(3):273-286. doi:10.4254/wjh.v11.i3.273
- Parke DV. Nutritional requirements for detoxification of environmental chemicals. Food Addit Contam. 1991;8(3):381-396. doi:10.1080/02652039109373987.
- Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chem Biol Interact. 2006;163(1-2):113-132. doi:10.1016/j.cbi.2006.05.010
- Halsted CH. B-Vitamin dependent methionine metabolism and alcoholic liver disease. Clin Chem Lab Med. 2013;51(3):457-465. doi:10.1515/cclm-2012-0308
- Al Garea MH, Alqasoumi AA, Alqahtani SA, Hadadi AH, Emara AM. Vitamin C as a potential ameliorating agent against hepatotoxicity among alcoholic abusers. Eur Rev Med Pharmacol Sci. 2023;27(8):3322-3335. doi:10.26355/eurrev_202304_32103
- Ozturk IC, Ozturk F, Gul M, Ates B, Cetin A. Protective effects of ascorbic acid on hepatotoxicity and oxidative stress caused by carbon tetrachloride in the liver of Wistar rats. Cell Biochem Funct. 2009;27(5):309-315. doi:10.1002/cbf.1575
- Bae S, Cho CH, Kim H, et al. In vivo consequence of vitamin C insufficiency in liver injury: vitamin C ameliorates T-cell-mediated acute liver injury in gulo(-/-) mice. Antioxid Redox Signal. 2013;19(17):2040-2053. doi:10.1089/ars.2012.4756
- Yu SJ, Bae S, Kang JS, et al. Hepatoprotective effect of vitamin C on lithocholic acid-induced cholestatic liver injury in Gulo(-/-) mice. Eur J Pharmacol. 2015;762:247-255. doi:10.1016/j.ejphar.2015.06.008
- Naziroğlu M, Cay M, Ustündağ B, Aksakal M, Yekeler H. Protective effects of vitamin E on carbon tetrachloride-induced liver damage in rats. Cell Biochem Funct. 1999;17(4):253-259. doi:10.1002/(SICI)1099-0844(199912)17:4<253::AID-CBF837>3.0.CO;2-R
- van Haaften RI, Haenen GR, Evelo CT, Bast A. Effect of vitamin E on glutathione-dependent enzymes. Drug Metab Rev. 2003;35(2-3):215-253. doi:10.1081/dmr-120024086
- Sen Gupta P, Karmakar S, Biswas I, et al. Vitamin E alleviates chlorpyrifos induced glutathione depletion, lipid peroxidation and iron accumulation to inhibit ferroptosis in hepatocytes and mitigate toxicity in zebrafish. Chemosphere. 2024;359:142252. doi:10.1016/j.chemosphere.2024.142252
- Tyczyńska M, Hunek G, Szczasny M, et al. Supplementation of micro- and macronutrients: a role of nutritional status in non-alcoholic fatty liver disease. Int J Mol Sci. 2024;25(9):4916. doi:10.3390/ijms25094916.
- Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005;26(4-5):391-404. doi:10.1016/j.mam.2005.07.002
- Liu J, Zhou ZX, Zhang W, Bell MW, Waalkes MP. Changes in hepatic gene expression in response to hepatoprotective levels of zinc. Liver Int. 2009;29(8):1222-1229. doi:10.1111/j.1478-3231.2009.02007.
- Yang X, Lv Y, Huang K, Luo Y, Xu W. Zinc inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in human hepatocytes (HepG2 cells). Food Chem Toxicol. 2016;92:17-25. doi:10.1016/j.fct.2016.03.012
- Zhang C, Ge J, Lv M, Zhang Q, Talukder M, Li JL. Selenium prevent cadmium-induced hepatotoxicity through modulation of endoplasmic reticulum-resident selenoproteins and attenuation of endoplasmic reticulum stress. Environ Pollut. 2020;260:113873. doi:10.1016/j.envpol.2019.113873
- Shen Y, Huang H, Wang Y, Yang R, Ke X. Antioxidant effects of Se-glutathione peroxidase in alcoholic liver disease. J Trace Elem Med Biol. 2022;74:127048. doi:10.1016/j.jtemb.2022.127048
- Xiao H, Parkin KL. Induction of phase II enzyme activity by various selenium compounds. Nutr Cancer. 2006;55(2):210-223. doi:10.1207/s15327914nc5502_13
- Indumathi MC, Swetha K, Abhilasha KV, et al. Selenium Ameliorates Acetaminophen-Induced Oxidative Stress via MAPK and Nrf2 Pathways in Mice. Biol Trace Elem Res. 2024;202(6):2598-2615. doi:10.1007/s12011-023-03845-3
- Gao PC, Chu JH, Chen XW, Li LX, Fan RF. Selenium alleviates mercury chloride-induced liver injury by regulating mitochondrial dynamics to inhibit the crosstalk between energy metabolism disorder and NF-κB/NLRP3 inflammasome-mediated inflammation. Ecotoxicol Environ Saf. Published online November 25, 2021. doi:10.1016/j.ecoenv.2021.113018
- Yang YZ, Liu ZH, Wang SC, et al. Magnesium isoglycyrrhizinate alleviates fructose-induced liver oxidative stress and inflammatory injury through suppressing NOXs. Eur J Pharmacol. 2020;883:173314. doi:10.1016/j.ejphar.2020.173314
- Zhao XJ, Yang YZ, Zheng YJ, et al. Magnesium isoglycyrrhizinate blocks fructose-induced hepatic NF-κB/NLRP3 inflammasome activation and lipid metabolism disorder [published correction appears in Eur J Pharmacol. 2021 Oct 15;909:174440. doi: 10.1016/j.ejphar.2021.174440]. Eur J Pharmacol. 2017;809:141-150. doi:10.1016/j.ejphar.2017.05.032
- Mehta R, Dedina L, O’Brien PJ. Rescuing hepatocytes from iron-catalyzed oxidative stress using vitamins B1 and B6. Toxicol In Vitro. 2011;25(5):1114-1122. doi:10.1016/j.tiv.2011.03.015
- Morris G, Anderson G, Dean O, et al. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. 2014;50(3):1059-1084. doi:10.1007/s12035-014-8705-x
- Uchida Y, Ferdousi F, Takahashi S, Isoda H. Comprehensive Transcriptome Profiling of Antioxidant Activities by Glutathione in Human HepG2 Cells. Molecules. 2024;29(5):1090. Published 2024 Feb 29. doi:10.3390/molecules29051090
- Yang K, Kim HH, Shim YR, Ryu T, Kim CW. Comprehensive transcriptomic analysis and meta-analysis identify therapeutic effects of N-acetylcysteine in nonalcoholic fatty liver disease. Front Pharmacol. 2023;14:1186582. Published 2023 May 15. doi:10.3389/fphar.2023.1186582
- de Andrade KQ, Moura FA, dos Santos JM, de Araújo OR, de Farias Santos JC, Goulart MO. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. Int J Mol Sci. 2015;16(12):30269-30308. Published 2015 Dec 18. doi:10.3390/ijms161226225
- Chang HS, Ko M, Ishizuka M, et al. Sodium 2-propenyl thiosulfate derived from garlic induces phase II detoxification enzymes in rat hepatoma H4IIE cells. Nutr Res. 2010;30(6):435-440. doi:10.1016/j.nutres.2010.06.007.
- Jung TS, Kim SK, Shin HJ, Jeon BT, Hahm JR, Roh GS. α-lipoic acid prevents non-alcoholic fatty liver disease in OLETF rats. Liver Int. 2012;32(10):1565-1573. doi:10.1111/j.1478-3231.2012.02857.x
- Hossain KFB, Akter M, Rahman MM, et al. Amelioration of Metal-Induced Cellular Stress by α-Lipoic Acid and Dihydrolipoic Acid through Antioxidative Effects in PC12 Cells and Caco-2 Cells. Int J Environ Res Public Health. 2021;18(4):2126. Published 2021 Feb 22. doi:10.3390/ijerph18042126
- Elshazly SM, El-Moselhy MA, Barakat W. Insights in the mechanism underlying the protective effect of α-lipoic acid against acetaminophen-hepatotoxicity [published correction appears in Eur J Pharmacol. 2014 Jun 15;733:102]. Eur J Pharmacol. 2014;726:116-123. doi:10.1016/j.ejphar.2014.01.042
- Ayyadurai VAS, Deonikar P, Fields C. Mechanistic understanding of D-glucaric acid to support liver detoxification essential to muscle health using a computational systems biology approach. Nutrients. 2023;15(3):733. doi:10.3390/nu15030733
- Jayasuriya R, Dhamodharan U, Ali D, Ganesan K, Xu B, Ramkumar KM. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine. 2021;92:153755. doi:10.1016/j.phymed.2021.153755
- Maher JM, Dieter MZ, Aleksunes LM, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46(5):1597-1610. doi:10.1002/hep.21831
- Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144-149. doi:10.4254/wjh.v6.i3.144
- Simón J, Casado-Andrés M, Goikoetxea-Usandizaga N, Serrano-Maciá M, Martínez-Chantar ML. Nutraceutical properties of polyphenols against liver diseases. Nutrients. 2020;12(11):3517. doi:10.3390/nu12113517.
- Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24(10):1423-1432. doi:10.1002/ptr.3207
- Muhammad I, Wang H, Sun X, et al. Dual Role of Dietary Curcumin Through Attenuating AFB1-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB1 Bioactivation and Detoxification. Front Pharmacol. 2018;9:554. Published 2018 May 25. doi:10.3389/fphar.2018.00554
- Wang H, Muhammad I, Li W, Sun X, Cheng P, Zhang X. Sensitivity of Arbor Acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of phase-II enzymes and Nrf2. Environ Toxicol Pharmacol. 2018;59:94-104. doi:10.1016/j.etap.2018.03.003
- Jayasuriya R, Dhamodharan U, Ali D, Ganesan K, Xu B, Ramkumar KM. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine. 2021;92:153755. doi:10.1016/j.phymed.2021.153755
- Yao HT, Li CC, Chang CH. Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats. Nutrients. 2019;11(8):1862. Published 2019 Aug 10. doi:10.3390/nu11081862
- Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46(4):1271-1278. doi:10.1016/j.fct.2007.10.006
- Wang Y, Wu J, Wang L, et al. Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins (Basel). 2022;14(12):876. Published 2022 Dec 15. doi:10.3390/toxins14120876
- You Y, Yoo S, Yoon HG, et al. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol. 2010;48(6):1632-1637. doi:10.1016/j.fct.2010.03.037
- Domitrović R, Jakovac H, Romić Z, Rahelić D, Tadić Z. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J Ethnopharmacol. 2010;130(3):569-577. doi:10.1016/j.jep.2010.05.046
- Liao GC , Jhuang JH , Yao HT . Artichoke leaf extract supplementation lowers hepatic oxidative stress and inflammation and increases multidrug resistance-associated protein 2 in mice fed a high-fat and high-cholesterol diet [published correction appears in Food Funct. 2021 Sep 20;12(18):8812. doi: 10.1039/d1fo90070f]. Food Funct. 2021;12(16):7239-7249. doi:10.1039/d1fo00861g
- Kirchhoff R, Beckers C, Kirchhoff GM, Trinczek-Gärtner H, Petrowicz O, Reimann HJ. Increase in choleresis by means of artichoke extract. Phytomedicine. 1994;1(2):107-115. doi:10.1016/S0944-7113(11)80027-9
- Kim M, Jee SC, Kim KS, Kim HS, Yu KN, Sung JS. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants (Basel). 2021;10(5):787. Published 2021 May 16. doi:10.3390/antiox10050787
- Ji LL, Sheng YC, Zheng ZY, Shi L, Wang ZT. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12-23. doi:10.1016/j.freeradbiomed.2015.03.035
- Chow HH, Garland LL, Hsu CH, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010;3(9):1168-1175. doi:10.1158/1940-6207.CAPR-09-0155
- Chupradit S, Bokov D, Zamanian MY, Heidari M, Hakimizadeh E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam Clin Pharmacol. 2022;36(3):468-485. doi:10.1111/fcp.12746
- Rom O, Liu Y, Liu Z, et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci Transl Med. 2020;12(572):eaaz2841. doi:10.1126/scitranslmed.aaz2841
- Mizota T, Hishiki T, Shinoda M, et al. The hypotaurine-taurine pathway as an antioxidative mechanism in patients with acute liver failure. J Clin Biochem Nutr. 2022;70(1):54-63. doi:10.3164/jcbn.21-50
- Jung YS. Metabolism of Sulfur-Containing Amino Acids in the Liver: A Link between Hepatic Injury and Recovery. Biol Pharm Bull. 2015;38(7):971-974. doi:10.1248/bpb.b15-00244
- Häussinger D, Graf D, Weiergräber OH. Glutamine and cell signaling in liver. J Nutr. 2001;131(9 Suppl):2509S-4S. doi:10.1093/jn/131.9.2509S
- Schemitt EG, Hartmann RM, Colares JR, et al. Protective action of glutamine in rats with severe acute liver failure. World J Hepatol. 2019;11(3):273-286. doi:10.4254/wjh.v11.i3.273
- Patzer JF 2nd. Thermodynamic considerations in solid adsorption of bound solutes for patient support in liver failure. Artif Organs. 2008;32(7):499-508. doi:10.1111/j.1525-1594.2008.00581.x
- Ayyadurai VAS, Deonikar P, Fields C. Mechanistic Understanding of D-Glucaric Acid to Support Liver Detoxification Essential to Muscle Health Using a Computational Systems Biology Approach. Nutrients. 2023;15(3):733. Published 2023 Feb 1. doi:10.3390/nu15030733
- Xu P, Dong S, Luo X, et al. Humic acids alleviate aflatoxin B1-induced hepatic injury by reprogramming gut microbiota and absorbing toxin. Ecotoxicol Environ Saf.









