Lindsay Goddard, MS, RDN, LD provides an overview of IgG Food testing and answers some frequently asked questions to assist with interpretations of results.
Why IgG?
When trying to heal the system, or relieve idiopathic symptoms, identifying food sensitivities can be a piece to the puzzle, or sometimes the direct cause. When IgG immune complexes are over produced due to a specific antigen, and unable to clear, they can cause significant inflammation throughout the body. IgG Food Sensitivity testing can be a simple and effective way to identify foods that are causing an inflammatory response without having to implement an elimination/reintroduction diet, which can take a significant amount of time and effort. This is especially true since reactions to foods can take up to several days to create noticeable symptoms. Furthermore, testing for IgG reactivity has numerous published case reports showing positive results with implementation.
What’s new about this test?
Prior to this, our laboratory used the long-accepted ELISA methodology, but when a substantially improved method becomes available, it is important to evolve. Our new XMAP technology increases the precision of identification by using intense signaling from fluorescents; the testing procedure is fully automated for greater precision and reliability, produces less waste by using multiplexed magnetic beads and requires less blood to run the sample for easier collection. We have also added specific allergens to problematic food categories, providing a more inclusive panel.
Why are Candida albicans and Saccharomyces cerevisiae on separate reports?
Yeasts’ primary antigens are not appropriate for protein-specific assays because their antigens are richer in glycans. Therefore, ELISA is the better methodology for assessing the immune response as it relates to yeasts, so sub-samples are run on separate equipment, generating a separate report. We are still able to use the same sample for both methods.
How does it compare to other food sensitivity testing?
There are several tests available, employing different methodologies, all claiming to be the best for food sensitivities. Below is a table that describes the differences, and how they compare with IgG.
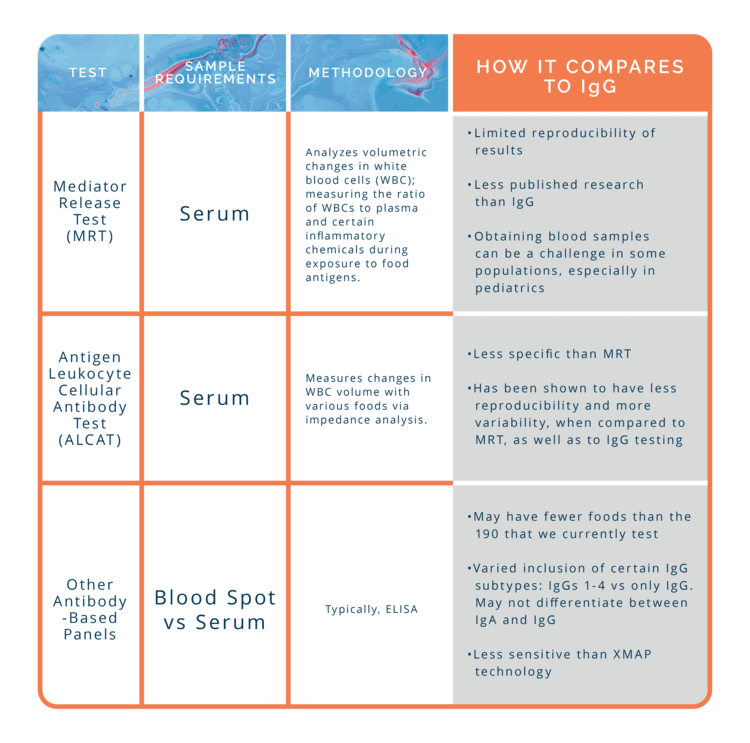
It should be noted that GPL includes all 4 subtypes of IgGs, to ensure more accuracy and we are only measuring IgG levels responses, not IgAs, since IgA is unable to bind complement and trigger generalized inflammation. To read more about why this is important, CLICK HERE
What is meat glue?
Meat glue is one of the new antigens on the test, interestingly enough, it is common for it to be elevated. Its source seems to be a bit obscure to some, but there may be more to the story than just an addition to processed foods. Meat glue is the transglutaminase enzyme, which can be derived from multiple sources: animals (vertebrates and invertebrates), plants, and bacteria (mainly the Streptoverticillium sp), the latter being the most common sourcing in commercial food products. Culinarily speaking, it is helpful in forming isopeptides between proteins, increasing viscosity, elasticity, firmness, and water-binding capacity in foods. The most common source of meat glue is in processed or imitation meat, cheese and other dairy products, and baked goods. Since it is considered vegan when derived from plants or bacteria, it may be in some plant-based products, as well. If you are trying to avoid meat glue, look for TG enzyme or TGP enzyme in the ingredients. Also be aware of manufactured meats, and meats described as reformed or formed. The USDA requires all companies disclose this ingredient on the food label, and there are no exemptions.
Transglutaminase is also involved in our biological chemistry. Transglutaminases are a family of enzymes categorized as transferases and mainly catalyze isopeptide bond formation, which can be difficult to degrade. You know, kind of like glue. They are found in several organs, and their specific functionality is dependent on their location: intracellular versus extracellular, as well as the type of tissue. For example, intracellular tissue transglutaminase plays an important role in apoptosis, while extracellular tissue transglutaminase can influence extracellular matrix stabilization, likely from its ability to bind fibronectin.
Although transglutaminase enzymes are diverse, they do have significance in several diseases. The disease most associated with this enzyme is celiac disease. Tissue transglutaminase cross links to gluten, stimulating a specific B-cell response that can result in production of anti-transglutaminase antibodies, which are measured as a part of the diagnosis of Celiac. The dysregulation that follows may impact the intestinal lining, causing the structural changes seen in Celiac disease. A high reactivity to meat glue on the IgG test is not diagnostic of Celiac Disease, but can provide justification of further testing of anti-transglutaminase antibodies if other factors are pointing to Celiac as well.
Why are there different derivatives of the same food?
First, it will be helpful to review how food proteins, which can serve as food antigens, bind to antibodies.
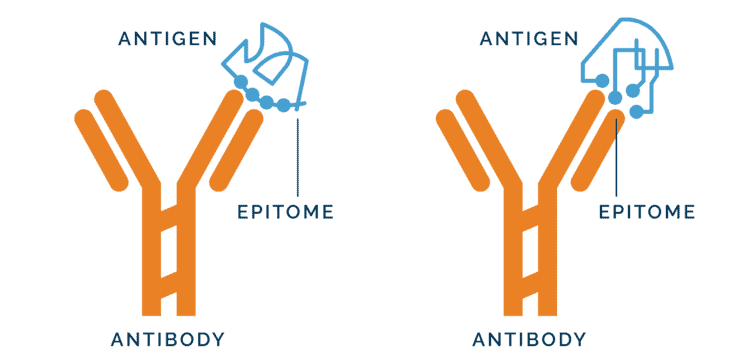
Adapted from Fundamentals and Methods for T- and B- Cell Epitope Prediction. Sanchez-Trincado JL, Gomez-Perosanz M, Reche PA. Journal of Immunology Research, vol. 2017
In this figure, we can see that on the antigen, the epitopes (i.e., where the antibody molecules dock) are very specific and end up with somewhat different structures, even though they may originally be from the same source. This is in part because protein structure can change significantly during food processing, potentially creating structures and epitopes so different they are not recognized by antibodies nor do they bind.
Measuring various components of a food allows for more detailed analysis as to which compound may be causing the inflammatory response, as well as increasing specificity and decreasing the chance of missing a potential source of inflammation. This not only pertains to the dairy section (cows’ and goats’ milk, casein, whey, beta-lactoglobulin, sheep’s yogurt, yogurt, mozzarella and cheddar cheese), but also gluten components (Wheat gluten, whole wheat, gliadin, barley, rye), and pineapple components (bromelain).
Does the casein differentiate between A1 vs A2 Casein?
No. The ability to differentiate between A1 and A2 caseins is not possible at this time.
If I stopped eating a particular food, and it is not showing up on the results as a problem, do I still need to remove it?
It can take up to 6 months after removing a food from the diet to not see an elevated IgG response to that particular food antigen. If a person is not consuming the food (or hasn’t done so in over 6 months), it likely will not trigger a large immune response, and will not show up as elevated on the test. A low reactivity to a certain food does not indicate that the person is now able to tolerate it or that the food can be safely reintroduced. It only means they have been vigilant in keeping it out of their diet. On the other hand, if the person has abstained from consuming a specific food and it does show positive on the test, then cross contamination with structurally similar proteins in the diet is likely the culprit.
Will the lipid derivative of foods on the IgG test be a problem?
The IgG response is to proteins, not lipids. However, people often question if they are sensitive to casein, can they consume butter? Or if they are sensitive to soy, can they consume soy lecithin? During the processing of extraction for these components, trace levels have been found of the corresponding protein, however, the protein is in such small amounts it is unlikely to be a problem. Most people seem to be able to tolerate it, though there are a select few who do not. An elimination/reintroduction phase may be helpful for further investigation in those rare cases.
Hopefully, this blog helps answer some of the common questions. If you still need help before ordering, you may contact customer service, or if you have a result that you need help interpreting, please schedule a consultation with the consultant staff.
Resources: 1. Sanchez-Trincado JL, Gomez-Perosanz M, Reche PA, "Fundamentals and Methods for T- and B-Cell Epitope Prediction", Journal of Immunology Research, vol. 2017, Article ID 2680160, 14 pages, 2017. SOURCE2. Kemeny DM, et al Sub-class of IgG in allergic disease. I. IgG sub-class antibodies in immediate and non-immediate food allergy. Clin Allergy. 1986; 16:571-81.3. Williams F. Use of the LEAP mediator release test to identify non-IgE mediated immunologic food reactions that trigger diarrhea predominant IBS symptoms results in marked improvement of symptoms through use of an elimination diet. American Journal of Gastroenterology: October 2004 – Volume 99 – Issue – P S277-S278. SOURCE.4. Lomangino, Kevin Food Sensitivity Testing: Evidence-Based Practice or Pricey Placebo?, Clinical Nutrition Insight: March 2013 - Volume 39 - Issue 3 - p 1-5 doi: 10.1097/01.NMD.0000428066.22177.25. Mullin, GE, Swift, KM, Lipski L, Turnbull LK, Rampertab SD. Testing for Food Reactions: The Good, the Bad, and the Ugly. Nutrition in Clinical Practice: April 2010-Volume 25-Issue 2-pg192-198 SOURCE6. Kieliszek M, Misiewicz A. Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol (Praha). 2014;59(3):241-250. doi:10.1007/s12223-013-0287-x7. United States Department of Agriculture, Food Safety and Inspection Service. Safety of Transglutaminase Enzyme (TG enzyme). 2017. SOURCE8. Sakly W, Thomas V, Quash G, El Alaoui S. A role for tissue transglutaminase in alpha-gliadin peptide cytotoxicity. Clin Exp Immunol. 2006 Dec;146(3):550-8. doi: 10.1111/j.1365-2249.2006.03236.x. PMID: 171007779. Patel S, Patel H. Technical Report: Understanding the Role of Dairy Proteins in Ingredient and Product Performance. U.S. Dairy Export Council. 2015. DMICMAIM5063_Dairy_Protein_Report_r6.pdf10. Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002 Dec 1;368(Pt 2):377-96. doi: 10.1042/BJ20021234. PMID: 12366374